Introduction
Direct RNA Sequencing (DRS) emerges as a revolutionary technological paradigm in genomic exploration, fundamentally transforming transcriptional analysis methodologies. Diverging from conventional RNA-sequencing techniques necessitating complementary DNA transformation, DRS facilitates immediate molecular interrogation of pristine RNA structures, meticulously preserving intricate genetic architectural elements and sophisticated post-transcriptional regulatory mechanisms.
This sophisticated approach equips scientific investigators with an unprecedented molecular lens, delivering profound insights into complex gene expression regulatory networks, intricate RNA modification patterns, and dynamic messenger RNA behavioral characteristics. By meticulously maintaining molecular fidelity and capturing sophisticated RNA structural nuances, DRS transcends traditional methodological constraints.
Principles of Direct RNA Sequencing
At the heart of DRS is nanopore sequencing technology. This method involves passing RNA molecules through a nanopore, where variations in electrical signals are detected as nucleotides translocate through the pore. Each nucleotide generates a unique electrical signal, allowing for the identification of individual bases and their modifications in real time. This single-molecule resolution enables researchers to detect multiple types of base modifications with remarkable precision.
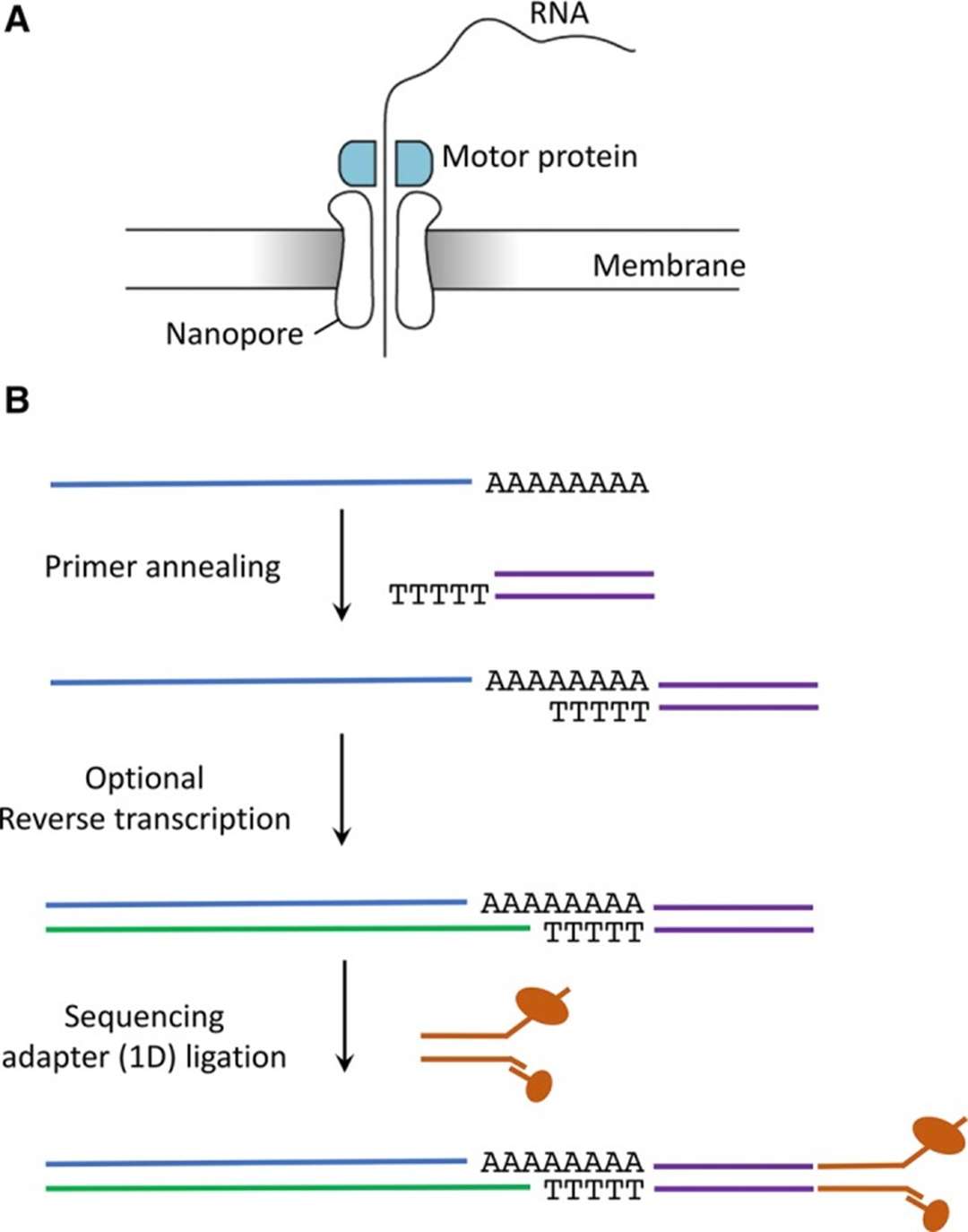 Figure1.Direct RNA sequencing library preparation steps.(Jonkhout, et.al. 2017)
Figure1.Direct RNA sequencing library preparation steps.(Jonkhout, et.al. 2017)
Nanopore Technology
Nanopore sequencing platforms, such as those developed by Oxford Nanopore Technologies (ONT), utilize a biological nanopore embedded in a membrane. As RNA strands pass through this nanopore, they disrupt an ionic current that flows across the membrane. The degree of disruption corresponds to specific nucleotides and their modifications. This real-time analysis allows for immediate insights into the RNA sequence and structural features without the need for extensive processing steps typically required by other sequencing methods.See more about the DRS protocol.
Advantages Over Traditional Methods
Traditional RNA sequencing methods often rely on short-read technologies that limit transcript analysis to approximately 50-100 base pairs. This restriction prevents thorough analysis of full-length transcripts and inhibits detailed studies of isoforms and alternative splicing events. In contrast, DRS can sequence long RNA molecules ranging from 70 to over 26,000 nucleotides in length, providing comprehensive information about entire transcripts.
Advantages and Challenges of Direct RNA Sequencing Technology
Advantages
- Avoidance of PCR Bias: One of the significant advantages of DRS is its ability to eliminate biases introduced during cDNA synthesis and PCR amplification. By directly sequencing RNA molecules, researchers can obtain accurate single-nucleotide resolution modification information from the reference transcriptome.
- Full-Length RNA Information: DRS allows for the direct sequencing of full-length mRNA transcripts, including poly(A) tails. This capability provides a more comprehensive view of transcript diversity and gene expression dynamics.
- Single-Nucleotide Resolution: The technology enables the detection of various base modifications at single-nucleotide resolution on individual mRNA molecules. This precision is crucial for understanding the functional implications of specific modifications on RNA behavior.
Challenges
- Signal-Modification Correlation: Despite its advantages, DRS faces challenges related to understanding how nanopore electrical signals correlate with modified bases in native mRNA. The incomplete knowledge in this area limits DRS's ability to detect diverse RNA modifications at the single-molecule level.
- Data Interpretation Complexity: The large volumes of data generated by DRS necessitate sophisticated bioinformatics analysis tools and expertise. Researchers must navigate complex datasets that require advanced computational methods for accurate interpretation.
Applications in RNA Modification Analysis
Overview of RNA Modification Types
Over 160 types of RNA modifications have been identified in nature, with key modifications such as N6-methyladenosine (m6A), 5-methylcytosine (m5C), N1-methyladenosine (m1A), and pseudouridine (Ψ) playing critical roles in various biological processes. These modifications are involved in regulating gene expression, mRNA stability, splicing, translation efficiency, and cellular response to environmental changes.
Biological Functions of Key Modifications:
- m6A: This modification is known to influence mRNA stability and degradation pathways. It plays a role in alternative splicing and translation efficiency.
- m5C: Associated with gene regulation, m5C impacts transcriptional activity and mRNA stability.
- m1A: This modification affects translation initiation and stability by influencing ribosome binding.
- Ψ: Pseudouridine enhances mRNA stability and translation efficiency by modifying base-pairing properties.
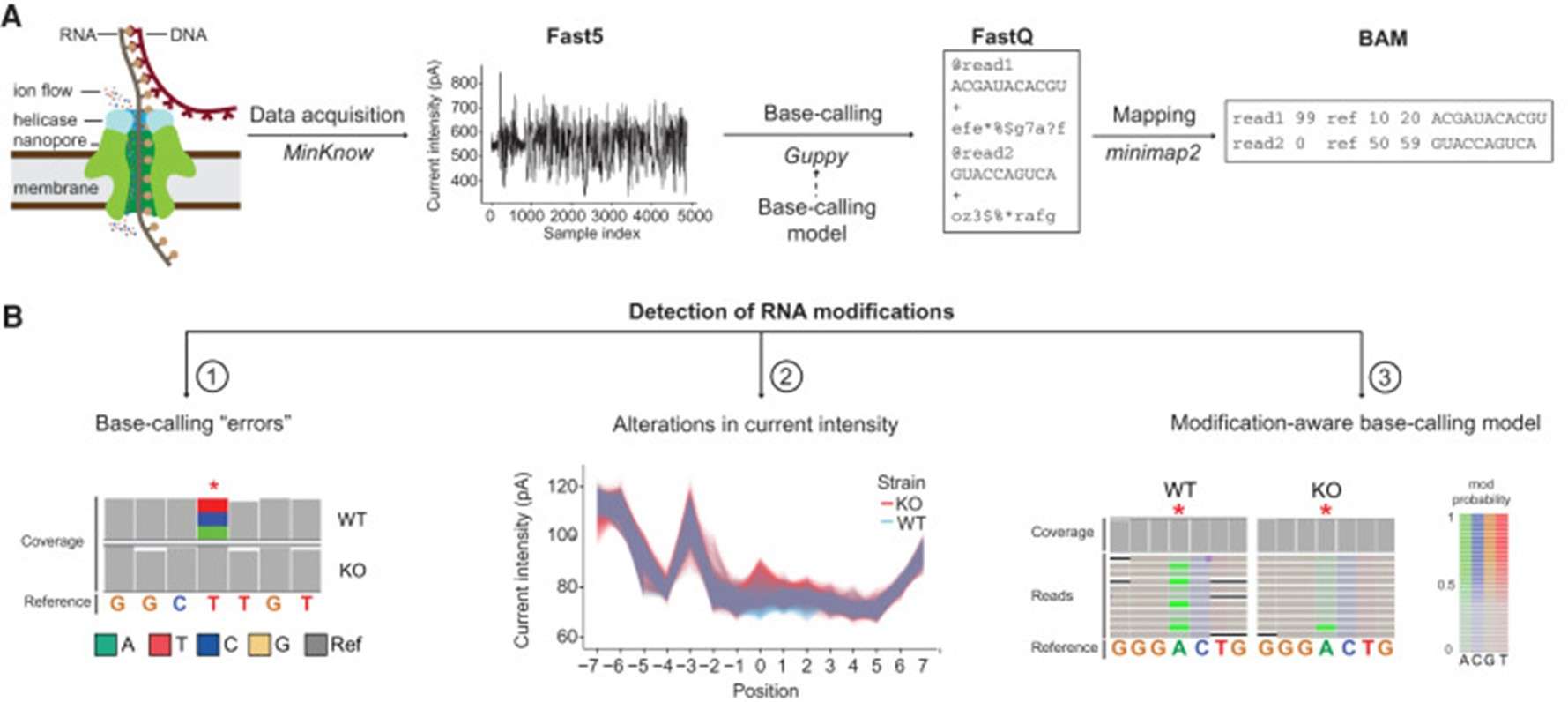 Figure2.An overview of using direct RNA sequencing to detect RNA modifications.(Begik, et.al. 2022)
Figure2.An overview of using direct RNA sequencing to detect RNA modifications.(Begik, et.al. 2022)
Existing Software for RNA Modification Detection in DRS
To facilitate the detection of RNA modifications using DRS data, several software tools have been developed:
- Nanopolish: A tool designed for analyzing nanopore sequencing data to identify modified bases.
- Tombo: A software package that utilizes statistical models to detect modified bases from raw nanopore signal data.
- MINES: A tool focused on inferring methylation states from nanopore sequencing data.
- Nanom6A: Specifically tailored for detecting m6A modifications in RNA sequences.
- m6Anet: A deep learning-based approach for predicting m6A sites using nanopore data.
These tools enhance detection accuracy by analyzing electrical signal patterns to infer modification sites on RNA molecules, providing new avenues for exploring the epitranscriptome.
Service you may intersted in
Resource
Development and Application of the TandemMod Transfer Learning Model
Application of Transfer Learning in DRS
The TandemMod model represents a significant advancement in applying transfer learning techniques to DRS data analysis. Developed by a research group led by Xiangchang et.al , this model enables simultaneous detection of multiple types of RNA modifications with high accuracy. By leveraging pre-trained models on large datasets, TandemMod can effectively generalize across different types of RNA sequences and modifications.
Creation of the In Vitro Epitranscriptomic (IVET) Dataset
To train and validate TandemMod effectively, researchers generated the In Vitro Epitranscriptomic (IVET) dataset through in vitro transcription of thousands of mRNA transcripts with diverse modification labels derived from a rice cDNA library containing T7 promoters. This accurately labeled dataset serves as a benchmark for DRS applications and provides essential training resources for subsequent machine learning models.
Construction of the Deep Learning Framework
The TandemMod model integrates advanced machine learning architectures including:
- One-Dimensional Convolutional Neural Network (1D CNN): Used for extracting features from raw electrical signal data.
- Bidirectional Long Short-Term Memory (Bi-LSTM): Captures long-range dependencies within sequences to improve prediction accuracy.
- Attention Mechanisms: Enhance model performance by focusing on relevant parts of input data when making predictions.
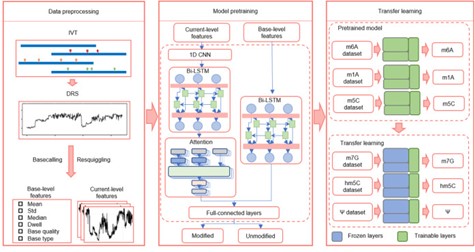 Figure3.Schematic of TandemMod model with data preprocessing, model pretraining and transfer learning.(Xiangchang , et.al. 2024)
Figure3.Schematic of TandemMod model with data preprocessing, model pretraining and transfer learning.(Xiangchang , et.al. 2024)
By utilizing electrical signal data corresponding to every five bases as input within this deep learning framework, TandemMod effectively handles complex datasets to predict RNA modification sites accurately.
Applications in Poly(A) Tail Analysis
Methods for Estimating Poly(A) Tail Length
Common software tools for estimating Poly(A) tail length include Nanopolish, Tailfindr, and Dorado. These tools can accurately estimate Poly(A) tail length in both reference-based and reference-free scenarios. This capability is critical for studying mRNA stability and translational efficiency.
Identification and Analysis of Poly(A) Sites
DRS technology enriches sequences containing poly(A) tails; over 90% of reads capture complete 3' end information. By aligning reads to a reference genome using tools like minimap2 or other alignment algorithms, researchers can precisely identify poly(A) sites. This capability offers new insights into the biological functions associated with poly(A) tails, including their roles in mRNA stability and translation initiation.
Applications in Routine Sequence Analysis
Transcript Identification and Fusion Gene Detection
DRS technology demonstrates significant advantages in transcript identification and fusion gene detection. By utilizing DRS methods, researchers can identify novel transcripts and fusion genes critical for understanding gene expression complexity—especially relevant in cancer research where fusion genes often play pivotal roles in tumorigenesis. For instance, studies have successfully identified novel fusion genes associated with specific cancer types using DRS technology. These findings have implications for targeted therapies that aim at disrupting oncogenic fusion proteins.
Alternative Splicing Analysis
DRS is also applied extensively in alternative splicing analysis, revealing previously uncharted complexities within gene expression profiles. Through DRS techniques, researchers can identify various splicing isoforms that provide valuable insights into gene function diversity and disease associations.In one study focusing on neurological disorders such as Alzheimer's disease, researchers utilized DRS to uncover alternative splicing events linked to disease progression—highlighting potential biomarkers for early diagnosis or therapeutic targets.
Transcript Quantification
Innovative software tools developed specifically for DRS applications—such as Bambu and NanoCount—offer solutions for precise transcript quantification. These tools enable accurate measurement of transcript expression levels across different conditions or developmental stages, facilitating advanced gene expression studies.A notable application involved quantifying differential gene expression levels between healthy tissues versus tumor tissues using DRS data—providing insights into tumor biology that could inform treatment strategies.
Future Developments in Direct RNA Sequencing Technology
Enhancements in Throughput and Accuracy
Recent iterations like the SQK-RNA004 kit from ONT have demonstrated higher accuracy rates (>94%) while producing significantly greater outputs (~30 million reads per PromethION flow cell). These improvements facilitate more extensive studies involving complex transcriptomes without compromising data quality or reliability 1.
Broader Acceptance Among Researchers
As nanopore direct RNA sequencing matures technically—addressing issues like basecall accuracy (>99%)—it is expected that broader acceptance among molecular biologists will accelerate further adoption within diverse research fields 2.
Applications Beyond Research Laboratories
The potential applications extend beyond academic research; industries such as pharmaceuticals may leverage DRS technologies for quality control processes—particularly relevant given recent developments concerning mRNA vaccines where accurate detection/modification assessments are critical 3.
Integration with Other Technologies
Future developments may also involve integrating DRS with other genomic technologies—such as CRISPR-Cas9 systems—to enhance precision editing capabilities while simultaneously monitoring transcriptomic changes resulting from genetic interventions
Conclusion
Direct RNA sequencing technology, with its unique advantages, is emerging as a standout in the field of multi-omics research. As the technology continues to evolve, it holds the promise of uncovering new mysteries of life, delivering deeper biological insights, and enabling more precise scientific solutions. The advancement of DRS is not only poised to propel fundamental scientific research but also to bring transformative changes to clinical medicine, drug development, and beyond.
References:
- Jonkhout, et.al. (2017). The RNA modification landscape in human disease. RNA (New York, N.Y.), 23(12), 1754–1769. https://doi.org/10.1261/rna.063503.117
- Begik, et.al. (2022). Exploring the epitranscriptome by native RNA sequencing. RNA (New York, N.Y.), 28(11), 1430–1439. https://doi.org/10.1261/rna.079404.122
- Xiangchang et.al. (2024). Transfer learning enables identification of multiple types of RNA modifications using nanopore direct RNA sequencing. Nature communications, 15(1), 4049. https://doi.org/10.1038/s41467-024-48437-4
- Zhong, et.al. (2023). Systematic comparison of tools used for m6A mapping from nanopore direct RNA sequencing. Nature communications, 14(1), 1906. https://doi.org/10.1038/s41467-023-37596-5


 Sample Submission Guidelines
Sample Submission Guidelines
 Figure1.Direct RNA sequencing library preparation steps.(Jonkhout, et.al. 2017)
Figure1.Direct RNA sequencing library preparation steps.(Jonkhout, et.al. 2017) Figure2.An overview of using direct RNA sequencing to detect RNA modifications.(Begik, et.al. 2022)
Figure2.An overview of using direct RNA sequencing to detect RNA modifications.(Begik, et.al. 2022) Figure3.Schematic of TandemMod model with data preprocessing, model pretraining and transfer learning.(Xiangchang , et.al. 2024)
Figure3.Schematic of TandemMod model with data preprocessing, model pretraining and transfer learning.(Xiangchang , et.al. 2024)