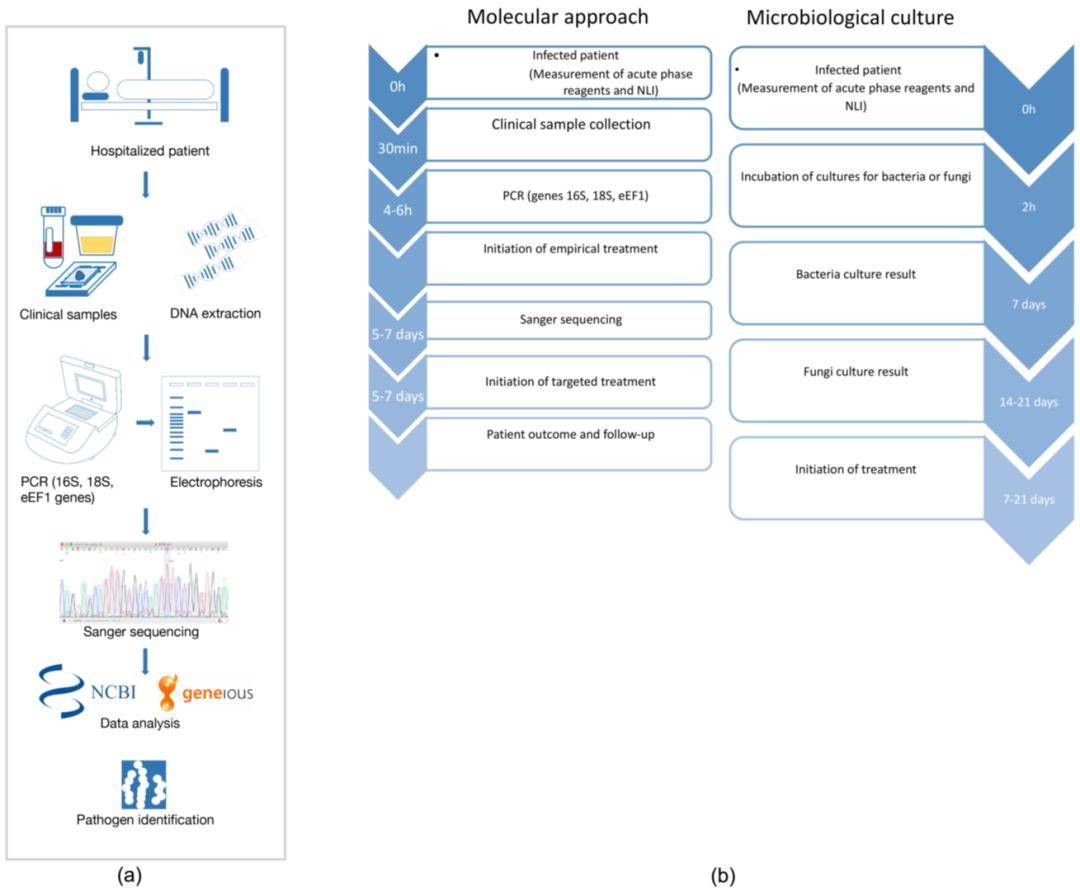
Phage Detection Methods Overview
In consideration of various detection targets, such as infectious phages, total Phages particles, nucleic acids, and proteins, this section provides a brief overview of prevalent Phages enumeration and detection methods, with a comparison of their merits and demerits outlined in Table 1.
Table 1. Comparison of Different Phages Enumeration and Detection Methods
| Method |
Basis of Detection/Enumeration |
Duration |
Manual Labor |
Cost |
Advantages |
Limitations |
Reference for Methodology |
| Double Agar Overlay Assay (DLA) |
Virulent phage particles |
1-2 days |
High |
$ |
Simple, effective, "gold standard," shows active virulence |
Slow, laborious, high standardization needed for precise reproducibility |
Kropinski et al., 2018 |
| Transmission Electron Microscopy (TEM) |
Magnification of virus particles |
2-3 days |
High |
$SS |
Works well with unknown phages |
Costly, laborious, high concentration needed |
Ackermann, 2012 |
| Flow Cytometry |
Viral particles |
4-12 hours |
Moderate |
$S$ |
Can detect different phages in a sample |
Expensive, low sensitivity, skilled operator needed |
Brussaard et al., 2000 |
| NanoSight |
Nanoparticle detection by laser-illuminated optical microscopy |
5-10 minutes |
Low |
$SS |
Rapid runtime |
Can be used only on clear, concentrated samples |
Anderson et al., 2011 |
| qPCR/RT-qPCR |
Viral nucleic acid |
2-6 hours |
Moderate |
$S |
Precise, reproducible |
Overestimation of virulent particles (one magnitude) |
Anderson et al., 2011 |
| Droplet Digital PCR (ddPCR) |
Viral nucleic acid |
2-6 hours |
Moderate |
$S |
No need for internal standards |
Could easily overestimate viral abundance |
Morella et al., 2018 |
| Mass Spectrometry |
Viral protein |
2-3 days |
High |
$SSS |
Accurate in determining PFU |
Time-consuming, surface protein mutants can give false results |
Wang et al., 2019 |
| Illumina Sequencing |
Viral nucleic acid library |
3-4 days |
Moderate |
$S$ |
Not well suited for quantification |
Significant amount of bioinformatics analysis needed |
Kumpp et al., 2012 |
| PacBio Sequencing |
Viral nucleic acid |
2-5 days |
Moderate |
$S$ |
Prone to sequencing errors |
Limited read length, higher error rate compared to other sequencing methods |
Kumpp et al., 2012 |
| NanoPore Sequencing |
Viral nucleic acid (can be amplified if needed) |
8-24 hours |
Moderate |
$S$ |
Compact, rapid, multiple uses |
Read errors, lower accuracy compared to other sequencing methods |
|
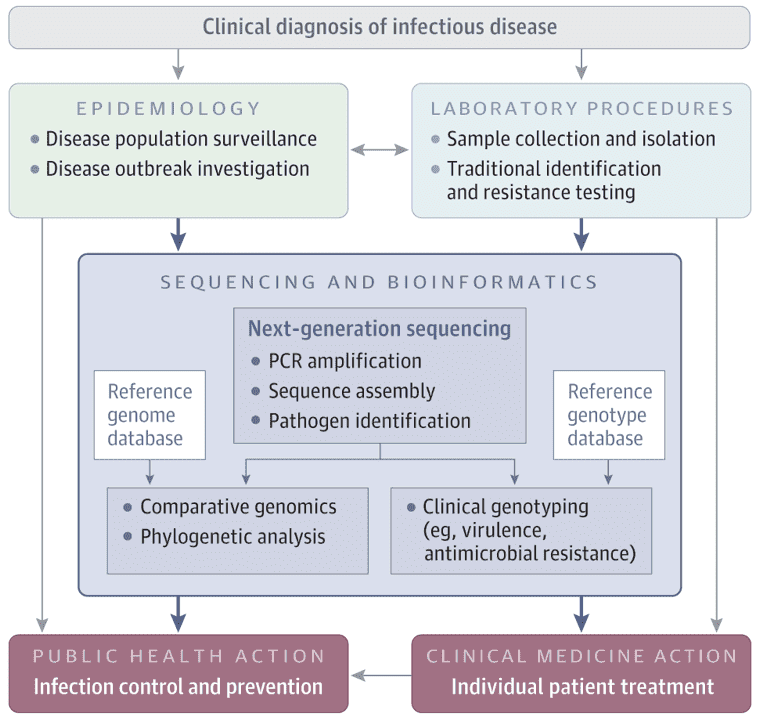
Detection Methods for Infectious Phages
Phages plaque counting stands as the recognized standard method for Phages enumeration. The Double Layer Agar (DLA) assay enables localized interaction between phages and host bacteria within the confined space of a dual agar layer (Petri dish). In this method, the bottom layer comprises a growth medium containing 1%-1.5% agar to support bacterial growth, while the top layer employs the same type of medium but with a lower agar concentration (0.4% to 0.6%), commonly referred to as soft agar. Subsequently, the top agar mixed with host bacteria is evenly spread over the bottom layer, forming a bacterial lawn. The sample containing phages is then gently dripped onto the top layer and allowed to air dry naturally, or alternatively, after co-incubation of phages and host bacteria, the mixture is combined with the top agar and uniformly spread over the bottom layer. Culturing is then carried out under appropriate temperature and time conditions conducive to bacterial growth.
Successful infection of the tested bacteria by phages results in the observation of distinct plaques or patches on the bacterial lawn. The formation of individual plaques arises from a single Phages initially lysing a host bacterium, followed by the progeny phages continuing the process of lysing adjacent bacteria. By sampling diluted samples on the bacterial lawn, the concentration of either the original Phages stock or infectious phages in the sample (i.e., titer) can be determined. Titer is typically expressed in plaque forming units (PFU, akin to colony forming units), as depicted in Figure 5.
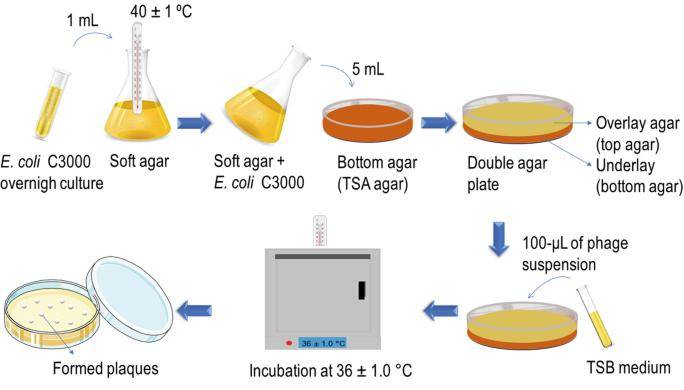 Figure 5. Double-Layer Plaque Assay Technique for Enumeration of Virus (Ruthchelly Tavares da Silva et al,. 2021)
Figure 5. Double-Layer Plaque Assay Technique for Enumeration of Virus (Ruthchelly Tavares da Silva et al,. 2021)
Detection Methods for Phages Particles
Transmission Electron Microscopy (TEM)
Transmission Electron Microscopy (TEM), an advanced microscopic technique, exhibits a resolution surpassing traditional optical microscopy by thousands of times, achieving 0.2 nanometers, thereby enabling the observation of viral fine structures. Despite the requisite for highly concentrated samples (10^6 particles per milliliter) to attain reliable experimental outcomes, this technique remains applicable for the quantitative analysis of viral particles. TEM demonstrates remarkable accuracy in identifying viral morphotypes and determining total counts. However, its application is somewhat constrained when handling multiple samples due to time-consuming operations and high costs. Furthermore, this technique is unsuitable for the analysis of complex samples, and the sample preparation process is intricate, necessitating skilled operators to handle complex instruments.
Flow Cytometry
Flow cytometry is another technique utilized for enumerating entire Phages particles. In this method, virus particles, labeled with fluorescent dyes, are guided through capillaries. The small diameter of the capillaries allows particles to flow in single file, enabling precise detection of light scattering signals induced by each viral particle. This technique finds widespread application due to its rapid and rigorous nature. It is noteworthy that fluorescence signals are unaffected by genome size, thus facilitating the quantitative assessment of phages.
Nucleic Acid and Protein Detection Methods for phages
Polymerase Chain Reaction (PCR)
Polymerase Chain Reaction (PCR) is a convenient and reliable technique centered on nucleic acid detection, which, compared to plaque assays, swiftly verifies the presence of phages. Utilizing Random Amplification of Polymorphic DNA (RAPD) PCR technology, distinguishing between different Phages lineages can be achieved within hours without the need for laborious whole-genome sequencing. However, the design of universal primers targeting Phages diversity proves particularly intricate due to the lack of universally conserved genes, such as the bacterial 16S rRNA gene. Additionally, PCR techniques entail limitations in quantifiable results primarily due to their endpoint nature.
Quantitative PCR (qPCR)
With the continuous advancement of scientific technology, Quantitative PCR (qPCR) has emerged. Similarly, significant progress has been made in protein detection, with Liquid Chromatography-Tandem Mass Spectrometry (LC-MS-MS) widely employed for precise detection and quantification of peptides in unknown samples.
In qPCR technology, the incorporation of fluorescent dyes enables the measurement of the quantity of DNA polymerized after each PCR cycle, thereby facilitating quantitative DNA detection. Fluorescent signals are real-time monitored by a thermal cycler, enabling simultaneous DNA amplification and fluorescence detection. Introduction of standard references allows for accurate calculation of initial DNA concentration. Common chemical components in qPCR platforms include fluorescent DNA dyes and short DNA probes labeled with fluorescent dyes and quencher molecules. Despite the selectivity of dye incorporation for double-stranded DNA quantification, probe-based assays are more precise. This is because a signal is only generated when successful hybridization and subsequent polymerization occur between the forward primer, reverse primer, and probe. Both methods are widely employed in Phages biology research.
Droplet Digital Polymerase Chain Reaction (ddPCR)
In Droplet Digital Polymerase Chain Reaction (ddPCR), samples are mixed with hydrophobic substances to form water-in-oil emulsions, with each droplet undergoing independent PCR reactions. Enumeration of amplified droplets by fluorescence detectors allows for the calculation of template DNA quantity based on the total number of droplets. This method enables quantification of initial concentration without external standards, demonstrating high accuracy and reliability.
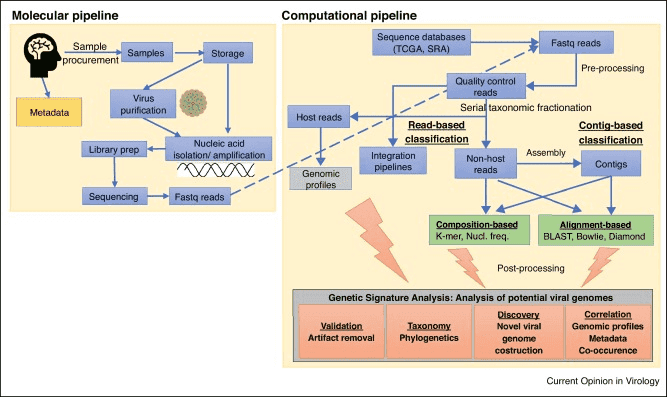
Whole Genome Sequencing (WGS)
Phage Whole Genome Sequencing (WGS) technology enables sequencing and assembly of complete Phages genomes without the need for prior phage isolation, through bioinformatic analysis. However, challenges persist in WGS as a counting and detection method due to factors such as the presence of bacterial host DNA, lack of reference databases for Phages genomes, and mosaic nature of Phages genomes. Nevertheless, WGS technology holds promise in identifying novel phages and detecting viruses in diverse habitats.
Quantification of Phages in Complex Samples
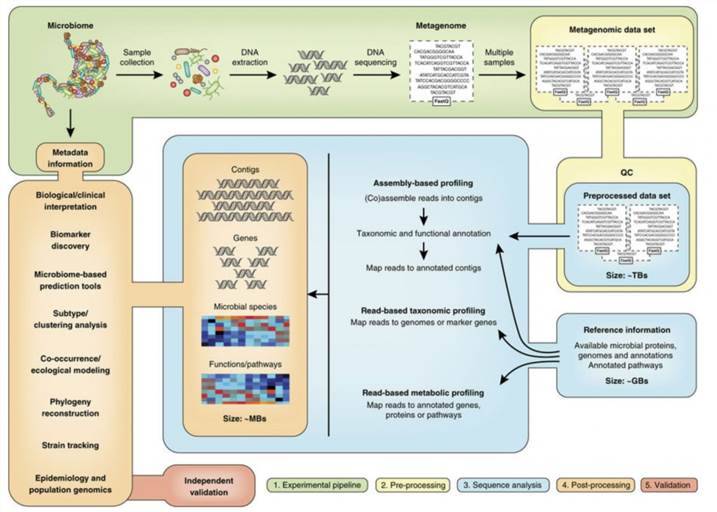
When dealing with complex samples such as clinical specimens, feces, or food, special processing measures must be implemented due to potential chemical compounds that may influence results. For quantification of individual phages in complex samples, an in-depth understanding of Phages and host characteristics is imperative to comprehensively grasp their dynamic features. Conversely, for quantifying entire Phages populations, the key lies in eliminating inhibitory factors. Generally, initial centrifugation aims to remove larger particles, followed by filtration using polytetrafluoroethylene membranes with apertures ranging from 0.22 μm to 0.45 μm to enrich virus particles in the filtrate. Subsequently, polyethylene glycol is utilized to concentrate the filtrate, obtaining a high concentration of viral suspension for subsequent counting and detection, such as employing DLA. Additionally, prior to employing PCR-based methods, physical separation is necessary to minimize residual PCR inhibitors. In utilizing whole genome sequencing technology for Phages quantification in complex samples, sequence reads must be filtered to remove non-viral sequences that may interfere with subsequent analysis.
Rational selection of preprocessing methods and downstream analysis techniques is crucial for obtaining comprehensive results regarding Phages concentration in complex samples. In recent years, with the widespread application of phages in clinical treatment of bacterial infections and food biopreservation, the demand for Phages counting and detection methods in complex samples has become increasingly urgent. Among these, the Double-Layer Agar Plate Assay (DLA) stands out as one of the most commonly used methods due to its rapid, economical, and accurate nature. Simultaneously, the continuous development of molecular biology techniques has enhanced the speed and repeatability of counting and detection. However, these methods are yet to differentiate infectious viral particles from defective ones, posing interpretation challenges compared to traditional DLA plaque analysis. In the future, integration of the latest advances in molecular and Phages biology holds the potential to combine the strengths of existing counting and detection methods, thus developing more advanced and efficient Phages counting and detection technologies.

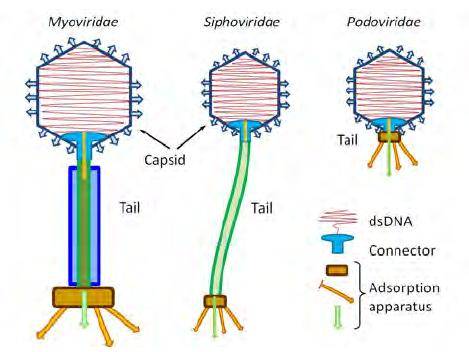 Figure 1. Tailed phage families (copyright of E.V. Orlova). E.V. Orlova 2012
Figure 1. Tailed phage families (copyright of E.V. Orlova). E.V. Orlova 2012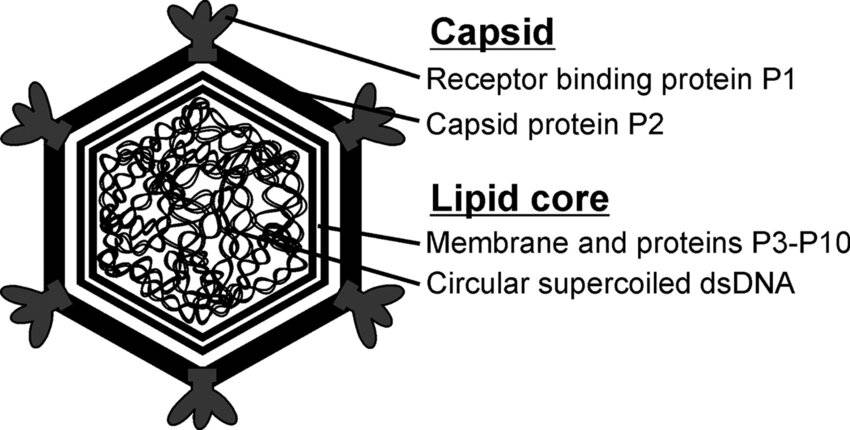 Figure 2. Schematic organization of bacteriophage PM2. (Hanna M Oksanen et al,. 2008)
Figure 2. Schematic organization of bacteriophage PM2. (Hanna M Oksanen et al,. 2008)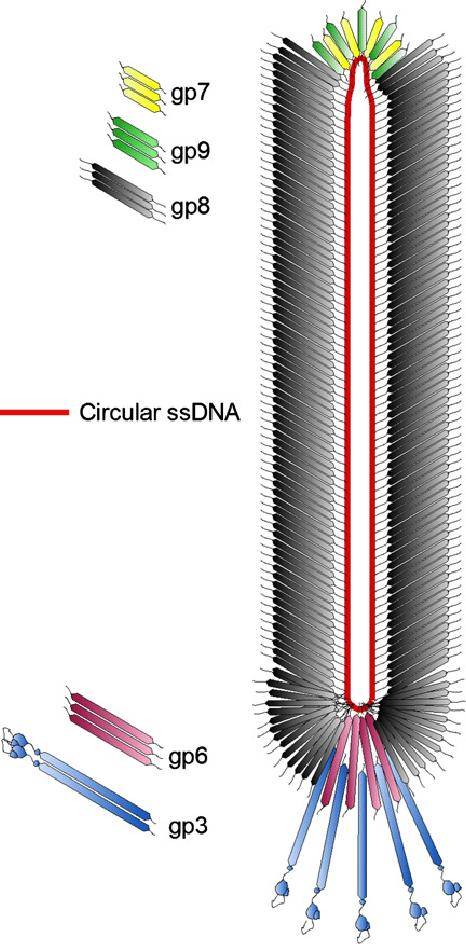 Figure 3. Schematic structure of bacteriophage M13. (Martin Ploß et al,. 2013)
Figure 3. Schematic structure of bacteriophage M13. (Martin Ploß et al,. 2013)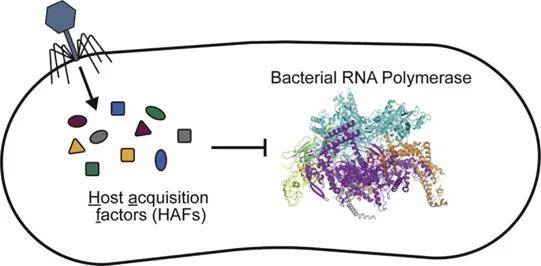 Figure 4. Phage-encoded small proteins (host acquisition factors, HAFs) regulate bacterial transcriptional machinery
Figure 4. Phage-encoded small proteins (host acquisition factors, HAFs) regulate bacterial transcriptional machinery Figure 5. Double-Layer Plaque Assay Technique for Enumeration of Virus (Ruthchelly Tavares da Silva et al,. 2021)
Figure 5. Double-Layer Plaque Assay Technique for Enumeration of Virus (Ruthchelly Tavares da Silva et al,. 2021)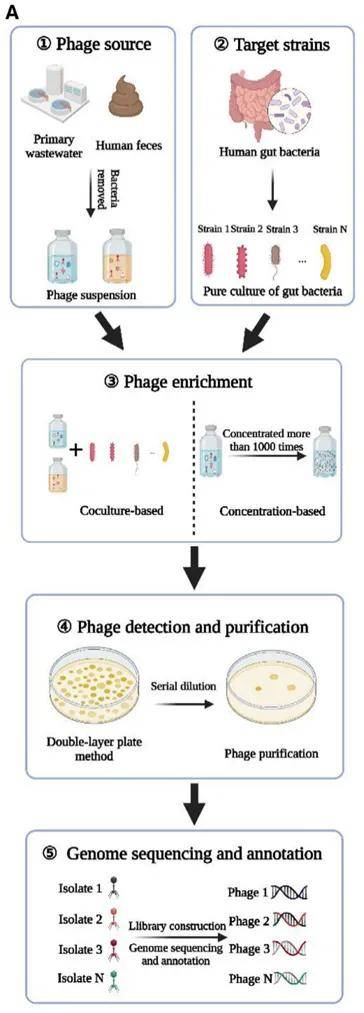 Figure 6. Phage isolation, culture and sequencing
Figure 6. Phage isolation, culture and sequencing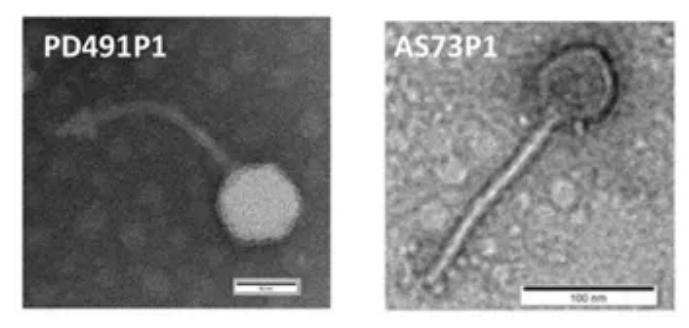 Figure 7. Electron micrograph of bacteriophage
Figure 7. Electron micrograph of bacteriophage