Next-Generation Sequencing (NGS) provides an effective means of analyzing complex eDNA samples directly and efficiently. Typically, it is necessary to analyze minute traces of DNA from various species within a sample without prior knowledge of species type or abundance. The sensitivity of NGS allows for the detection of extremely low concentrations of eDNA in the environment. By utilizing NGS, researchers can simultaneously analyze thousands of species within a single eDNA sample, which helps reveal the full diversity information contained in ecological samples. Consequently, eDNA NGS is becoming a powerful tool for studying ecosystem biodiversity and has proven to be an effective biological monitoring method.
eDNA sequencing encompasses several methods, with eDNA metabarcoding being one of the most commonly used techniques. Each species possesses a unique DNA sequence, often referred to as a barcode. This DNA barcode consists of highly variable regions between conserved genomic regions. eDNA metabarcoding involves the targeted amplification and sequencing of these barcodes, typically focusing on mitochondrial cytochrome c oxidase I (COI) or the 18S ribosomal subunit. This approach is effective for distinguishing higher eukaryotes.
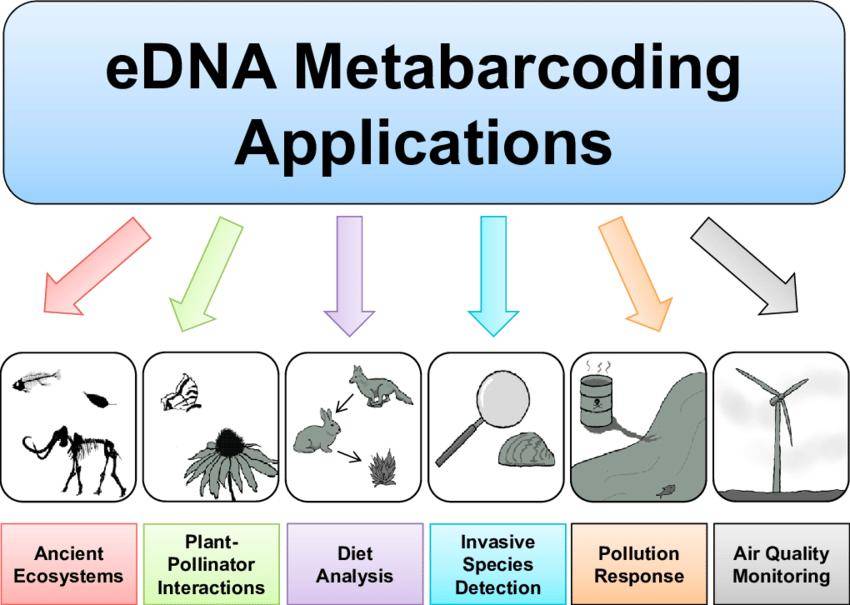 Figure 1 Applications of environmental DNA metabarcoding in aquatic and terrestrial ecosystems. (Krista M. Ruppert et al,. 2019)
Figure 1 Applications of environmental DNA metabarcoding in aquatic and terrestrial ecosystems. (Krista M. Ruppert et al,. 2019)
Metagenomics of Environmental Samples
Environmental metagenomics enables the analysis of entire microbial communities within complex environmental samples. This approach predominantly relies on sequencing the 16S ribosomal RNA (rRNA) gene for bacterial identification and the Internal Transcribed Spacer (ITS) region for fungal detection. Both 16S and ITS rRNA gene sequencing are established methodologies for comparative phylogenetic analysis and environmental sample classification.
The 16S rRNA gene sequencing method provides a comprehensive means to identify bacterial taxa, while ITS rRNA gene sequencing is utilized for detecting and characterizing fungal species. These techniques facilitate the taxonomic classification and phylogenetic comparison of microbial communities, enabling researchers to discern microbial diversity and functional potential within various environments.
In addition to targeted rRNA gene sequencing, shotgun metagenomic sequencing of eDNA is employed to study species that may be present in high abundance within a sample, such as bacteria or small eukaryotes. Shotgun sequencing provides a broader and more detailed analysis by capturing the entire genomic content of the sampled microorganisms, thus offering insights into community structure, gene function, and metabolic pathways.
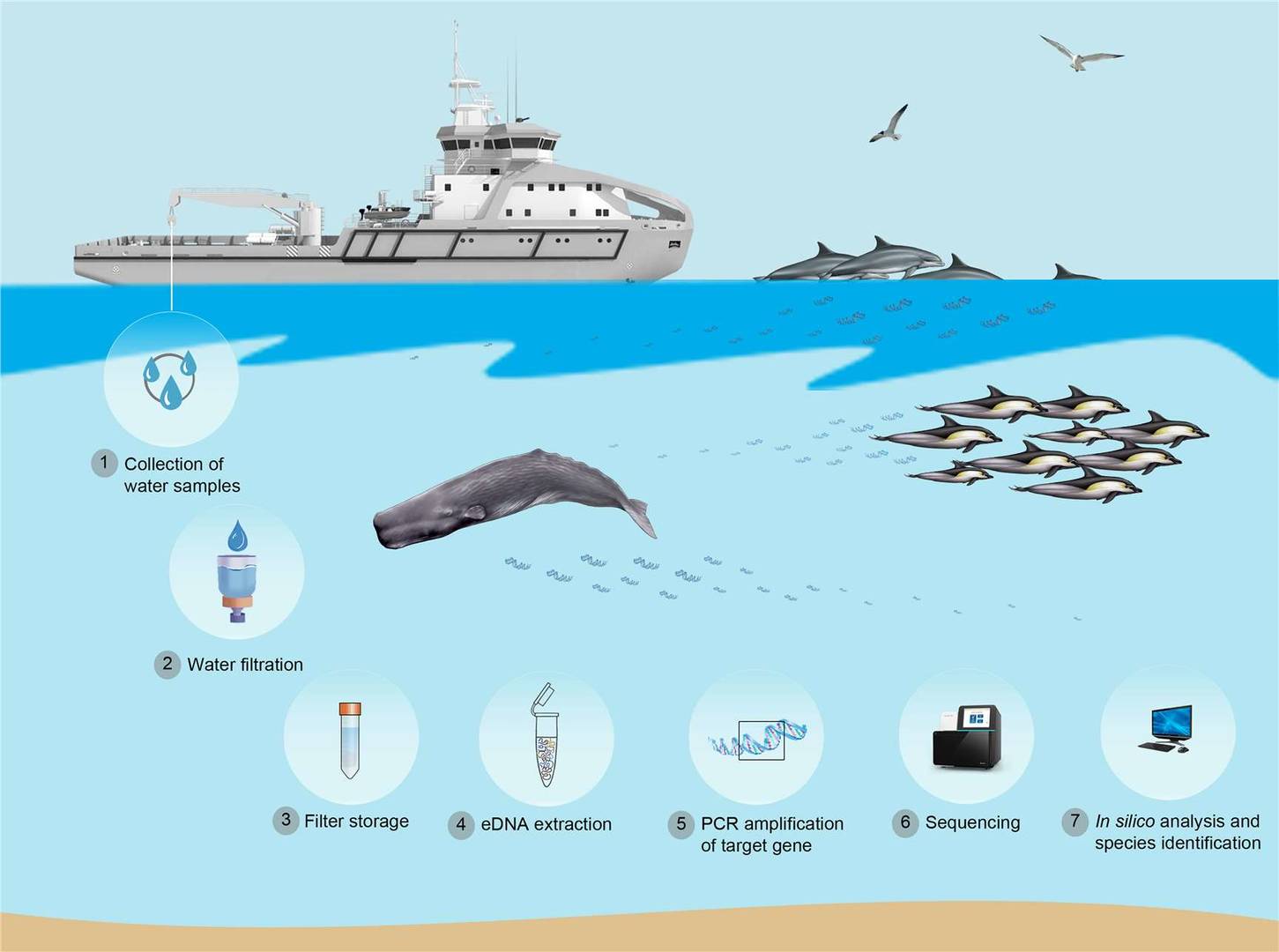 Figure 2 Application of eDNA methodology for marine mammals monitoring (Paula Suarez-Bregua et al,. 2022)
Figure 2 Application of eDNA methodology for marine mammals monitoring (Paula Suarez-Bregua et al,. 2022)
The Role of Environmental DNA (eDNA) in Ecosystem Monitoring
Importance of Environmental Health
The environment is crucial for various aspects of human life, including community well-being, recreational activities, drinking water, and transportation. Beyond changes in climate, human activities increasingly impact environmental health. As such, monitoring and maintaining environmental quality are essential for sustaining these vital services.
eDNA as a Diagnostic Tool
eDNA analysis serves as a "clinical" diagnostic tool for assessing environmental health. This method provides a window into the intricate details of ecosystems, allowing for an in-depth examination of environmental conditions. By leveraging genomic techniques, eDNA offers a critical data source for informed decision-making.
For example, by collecting and concentrating several liters of seawater from coral reefs, researchers can detect over 80 fish species from the residual DNA present in the sample. Sampling across different regions and time points facilitates the observation of changes in species diversity, thereby enabling the assessment of the impacts of various factors or activities on ecosystems. This approach represents a novel form of liquid biopsy, providing valuable insights into environmental dynamics and health.
Solutions in eDNA Sequencing and Metagenomic Next-Generation Sequencing (mNGS)
Similar Challenges
eDNA sequencing and metagenomic next-generation sequencing (mNGS) of human pathogens encounter numerous analogous challenges and solutions. A common issue is the predominance of bacterial DNA in environmental samples. For instance, when analyzing one liter of seawater or one gram of soil, more than 99% of the DNA typically derives from bacteria. To address this, selective enrichment methods must be employed to target specific organisms while excluding unwanted DNA.
DNA Extraction Difficulties
Another significant challenge is the extraction of DNA. Effective extraction methods must be tailored to different sample types to ensure that low-abundance species can be detected. Without optimized extraction protocols, the detection of these less prevalent organisms may be compromised.
Data Analysis and Quantification
A further issue pertains to the relationship between the eDNA extracted from a sample and the actual number of organisms present. The correlation between eDNA presence and organismal abundance remains uncertain. This is due to the variability in the rate at which organisms release DNA into the environment, making precise quantification problematic.
Database and Analysis Limitations
The accuracy of DNA barcoding in identifying species depends heavily on comprehensive and robust reference databases. The development of such databases requires collaborative efforts from researchers worldwide. The data analysis methods for eDNA are still evolving. Many approaches exist for interpreting eDNA data, but the challenge lies in distinguishing true signals from background noise while maintaining the sensitivity needed to detect rare taxa.
Whether as a novel liquid biopsy method or an advanced version of mNGS, eDNA sequencing represents a cutting-edge domain in genomics. It offers promising advancements for environmental research, monitoring, and conservation, providing a more efficient and timely tool for assessing and protecting ecological systems.
Advantages of eDNA Sequencing Service
High Sensitivity and Detection Accuracy : Detects species at low densities or elusive ones, crucial for identifying cryptic, endangered, or invasive species.
Non-Invasive and Ethical: No need for direct contact with organisms, minimizing disturbance to wildlife and habitats.
Cost-Effective and Efficient: Simplifies fieldwork and reduces costs by detecting multiple species from a single sample.
Broad Detection Spectrum: Can detect a wide range of organisms, including microorganisms, plants, and animals, from various samples like water, soil, and sediment.
Reduced Observer Bias: Standardized molecular techniques ensure consistent and objective results, reducing variability from observer expertise.
Legal and Regulatory Compliance: Often does not require permits for sampling, making it easier to conduct studies in protected or regulated areas.
Data for Long-Term Monitoring: Suitable for ongoing monitoring, tracking changes in species over time for conservation and environmental studies.
Enhanced Field Safety: Allows for safer fieldwork during daylight and favorable weather, reducing risks in hazardous environments.
Ability to Archive Samples: Samples can be archived for future reanalysis, enabling retrospective studies and validation of past research.
Supporting Sustainable Practices: Reduces the environmental footprint of research by minimizing physical specimen collection and promoting eco-friendly lab practices.
Our eDNA Analysis and Sequencing Service Workflow
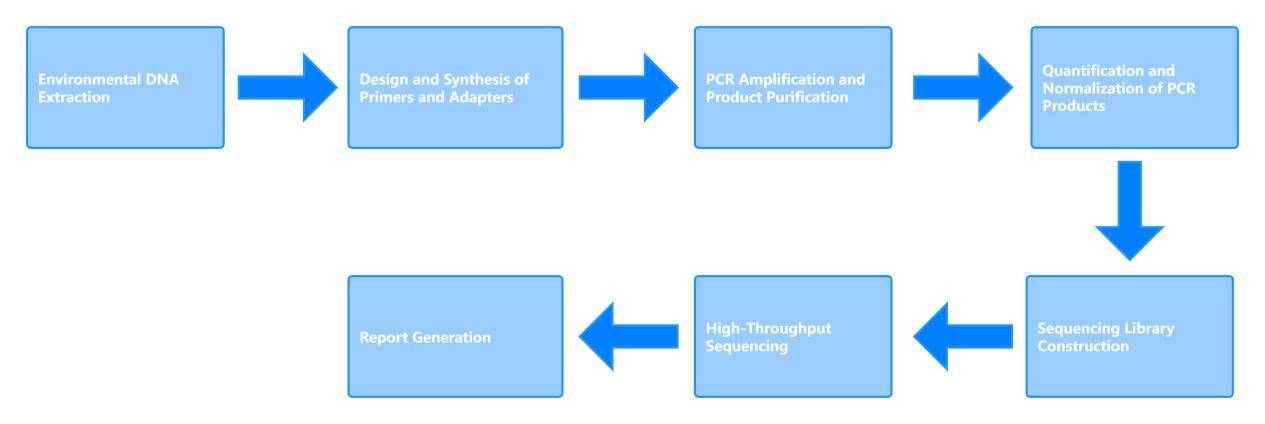
Sample Requirements for Environmental DNA Analysis
Importance of Sample Quality
High-quality data in eDNA analysis is contingent upon the integrity of the samples, which in turn relies on standardized sampling procedures. Given the diversity of eDNA sample types, the methods for sample collection vary accordingly. Furthermore, the abundance and diversity of microorganisms can be influenced by sampling, handling, and storage conditions. Improper procedures can adversely affect the results.
Sample Quality Specifications
For eDNA samples to be deemed acceptable, the following quality criteria must be met:
| Product |
Concentration |
Nucleic Acid Amount |
Notes |
| eDNA |
≥10 ng/µl |
≥200 ng |
DNA should exhibit a distinct main band, free from degradation, RNA, proteins, or other contaminants. OD260/280 should be between 1.8 and 2.0, and OD260/230 should be ≥1.0. |
Recommended Sample Quantities
Different types of samples require specific quantities for optimal analysis. The recommended amounts are as follows:
| Sample Type |
Suggested Quantity |
| Fecal Samples |
≥100 mg |
| Water (Filter Membranes) |
≥2 filters |
| Soil |
≥1 g |
| Activated Sludge, Sediments |
≥3 g |
| Air Samples |
≥2 filters |
| Surface Samples |
≥500 mg |
Sample Preparation Recommendations
1. Fecal Samples
Fecal specimens must be collected utilizing sterile fecal collection devices or other approved sterilized containers. To ensure optimal sample integrity, collection should focus on the central portion of the feces, as the external surface is likely to contain exfoliated intestinal epithelial cells and is susceptible to environmental contamination. Furthermore, exposure to atmospheric oxygen can result in the degradation of bacterial DNA. Upon collection, samples should be promptly transferred into sterile centrifuge tubes and subsequently stored at -80°C to preserve their viability until further analysis.
2. Water (Filter Membranes)
The sampling depth and range of water should be determined based on the research objectives. Filters with appropriate pore sizes should be used to collect samples. For sample processing, water can be filtered using a filtration pump or by direct filtration until visible coverage is achieved on the filter membrane. Filters should be collected for DNA extraction or stored at -80°C.
Note: Due to potential transportation issues, it is not recommended to send water samples directly.
3. Soil
General Soil: Determine the sampling area based on research needs. All sampling tools should be sterilized beforehand. During sampling, surface debris should be removed, and soil should be collected from the desired depth (e.g., 5–20 cm). Multiple samples should be combined and homogenized. Transfer 2–10 g of the mixed soil into sterile centrifuge tubes and store at -80°C.
Rhizosphere Soil: Large soil clumps around the roots should be removed, and loosely attached soil should be discarded. Collect tightly adhered soil from the roots using sterile brushes or similar tools, transferring 2–10 g into sterile centrifuge tubes. Store at -80°C.
Note: Collectors should wear disposable gloves and masks to minimize contamination. Self-sealing bags are not recommended for sampling to prevent cross-contamination.
4. Activated Sludge and Sediment Samples
Activated Sludge: Collect a sample of suspended sludge greater than 10 ml (approximately 5–10 g of sediment) from the activated sludge system using sterile centrifuge tubes. Store at -80°C.
Note: If the sediment volume is insufficient, increase the sampling quantity.
Sediment: Collect 5–10 g of sediment from a depth of 0–10 cm from the water bottom or surface, depending on the research purpose. Use sterile sampling bags or centrifuge tubes and store at -80°C.
5. Air Samples
Select sterile filters with appropriate pore sizes based on research needs. Air should be drawn through the filters until visible coverage is achieved. Given the low microbial content in air samples, longer sampling times are recommended to increase the likelihood of capturing a diverse microbiome. Transfer the filters into sterile cryovials or centrifuge tubes, freeze in liquid nitrogen, and store at -80°C.
6. Surface Samples
Place the collected object into a 50 ml sterile centrifuge tube and add 1x PBS buffer. Vortex the mixture for at least 2 hours to allow microorganisms to detach from the surface and aggregate in the PBS buffer. Centrifuge the buffer at 4°C, 12,000 g for 10 minutes, and store the pellet at -80°C. Alternatively, filter the buffer through a 0.22 μm filter membrane, place the filter in a sterile centrifuge tube, and store at -80°C.
7. Swab Samples
Insert the swab into the target area, gently rotate 3–5 times, and then slowly withdraw. Collect 2–3 swabs. Place the swabs into sterile cryovials or cut the swab heads into the cryovials. Seal tightly and immediately place in an insulated box with ice or ice packs. Store at -80°C.
Sample Shipping Recommendations
Dry Ice Quantity: Calculate the required amount of dry ice using the formula: Required dry ice (kg) = Transport time (days) * 5 (kg/day). It is advisable to use both powder and block dry ice.
Sample Placement: Line the bottom of the foam container with dry ice, place the samples covered with sealing film, and cover with additional dry ice. Fill the container with bubble wrap, absorbent paper, or other cushioning materials.
Sample Packaging: To prevent sample contamination due to packaging material rupture during transport, use centrifuge tubes for packaging. Ensure that the contents do not exceed 70% of the tube's volume to avoid rupture.
FAQs
Frequently Asked Questions (FAQ)
- 1. Can eDNA detect invasive species?
- 2. How is eDNA used in tracking endangered species?
- 3. What role does eDNA play in assessing water quality?
- 4. Can eDNA be used to monitor soil and sediment health?
- 5. What are the advantages of using eDNA in environmental monitoring?
- 6. What Types of Samples Can Be Collected?
- 7. How Quickly Can Results Be Obtained?
- 8. How Does CD Genomics Ensure Data Quality?
- 9. Why Choose CD Genomics for eDNA Analysis?
- 10. What Will the Results Look Like?


 Figure 1 Applications of environmental DNA metabarcoding in aquatic and terrestrial ecosystems. (Krista M. Ruppert et al,. 2019)
Figure 1 Applications of environmental DNA metabarcoding in aquatic and terrestrial ecosystems. (Krista M. Ruppert et al,. 2019) Figure 2 Application of eDNA methodology for marine mammals monitoring (Paula Suarez-Bregua et al,. 2022)
Figure 2 Application of eDNA methodology for marine mammals monitoring (Paula Suarez-Bregua et al,. 2022)