The global dissemination of antimicrobial resistance has become a significant concern, prompting widespread efforts to develop effective detection techniques for antibiotic resistance genes (ARGs). The ability to trace the migration and transmission of ARGs across environmental, plant, animal, and human populations is crucial for understanding and mitigating the spread of resistance. This review outlines the historical development of nucleic acid-based detection technologies, with a focus on their initial application to ARGs detection. It categorizes the technologies based on their principles, advantages, disadvantages, and potential for future development. Additionally, it proposes the development of an in situ rapid detection technique for ARGs, integrating isothermal amplification with CRISPR/Cas technology.
Development and Classification of ARGs Detection Technologies
The timeline of the invention of various detection technologies is systematically organized. The invention of isotope-labeled in situ hybridization (ISH) marked the rise of modern nucleic acid molecular biology-based detection methods. From 1990 to 2010, a surge of new technologies emerged, driven both by optimizations of existing methods, such as advancements in PCR and sequencing technologies, and by the continuation of the PCR approach, which led to the exploration of new primer design strategies and functional enzymes for various forms of isothermal amplification.
By the 1990s, these technologies began to be applied to the detection of ARGs, with Sanger sequencing and PCR serving as the pioneers for detecting sulfonamide and aminoglycoside resistance genes. The period between 2000 and 2010 represents a peak in the exploration of ARG detection methods, reflecting the lag between the invention and application of these technologies. This period also signifies a growing recognition of the importance of antibiotic resistance. The preferences for detecting specific target genes, such as mecA, rpoB, and β-lactamase-encoding genes, provided valuable reference points for subsequent applications of new technologies.
Based on detection principles and the extent of their adoption, the nucleic acid detection technologies are divided into four major categories: the gold standard of detection, PCR and its derivatives; comprehensive detection technologies, such as gene sequencing; emerging technologies, including isothermal amplification and CRISPR/Cas systems; and other fluorescence-based strategies. A more detailed discussion of each category's technological developments, principles, advantages, disadvantages, and current applications is presented in the following sections.
Gold Standard of Detection – PCR and Derivative Techniques
Polymerase chain reaction (PCR) and its derivatives have become extensively utilized in the detection of ARGs. The underlying principle of PCR is the exponential amplification of a target sequence through a series of thermal cycles, which include denaturation, annealing, and elongation steps facilitated by DNA polymerase. When combined with downstream characterization methods such as gel electrophoresis, fluorescent dyes, or fluorescence probes, PCR enables both qualitative and quantitative analysis of target genes.
Quantitative PCR (qPCR) ARGs detection is a representative technology, which measures the concentration of a target in a sample by correlating the intensity of the fluorescent signal. Due to its ability to detect target concentrations as low as a single copy per microliter in approximately two hours, qPCR has become the gold standard in ARG detection. To further improve sensitivity, droplet digital PCR (ddPCR) was developed, offering a detection limit an order of magnitude lower than that of qPCR. High-throughput qPCR was subsequently introduced to increase detection throughput. In its initial application to ARGs detection, high-throughput qPCR utilized 295 primer pairs to simultaneously quantify aminoglycoside and β-lactam ARGs in soil samples from a park irrigated with reclaimed water.
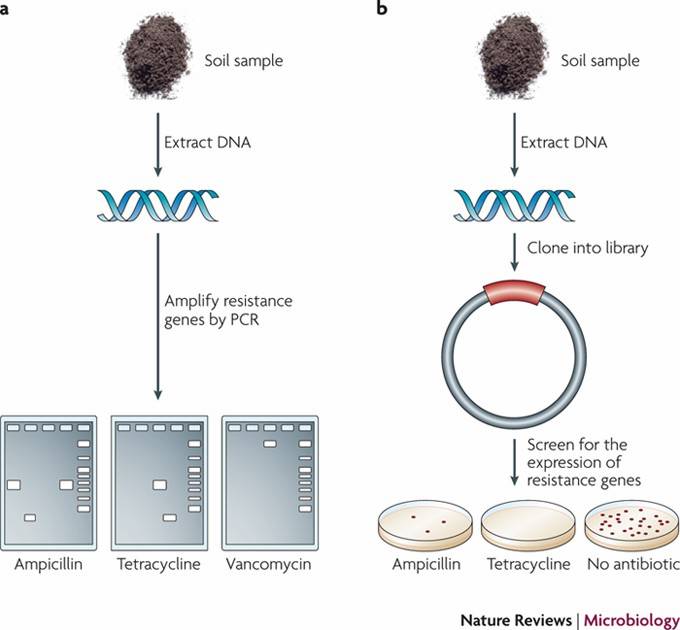 Detecting antibiotic genes in natural samples.a A PCR-based approach. b Functional metagenomics. (Heather K Allen et al,.2010)
Detecting antibiotic genes in natural samples.a A PCR-based approach. b Functional metagenomics. (Heather K Allen et al,.2010)
Comprehensive Detection Technology – Gene Sequencing
Sequencing technologies provide a distinct advantage in the screening of ARGs due to their capacity to read all bases within a sample. The core principle of sequencing is the identification of nucleic acid sequences through fluorescence or electrochemical signals corresponding to individual nucleotides. The Sanger method, recognized as first-generation sequencing, balances precision with cost-effectiveness. It was successfully employed as early as 1990 for the detection of the sulfonamide resistance gene sul3 in Mycobacterium fortuitum.
Next-generation sequencing (NGS) significantly increased throughput and enabled metagenomic analysisof complex environmental samples, allowing for the annotation of hundreds to thousands of ARGs. Third-generation sequencing, distinguished by its long-read capabilities, retains extensive upstream and downstream genetic information. For instance, PacBio SMRT technology has an average read length of 13.5 kb, while Oxford Nanopore sequencing can achieve read lengths exceeding 4 Mb. Sequencing technologies offer a unique advantage in non-targeted screening of ARGs, but their application is constrained by limitations such as long detection and analysis times, as well as challenges in achieving absolute quantification.
Table 1. Comparison of three sequencing technologies. (Aljuboori M. Nafea et al,.2024)
|
First generation sequencing
(Sanger method) |
Second generation
sequencing(NGS
method) |
Third generation
sequencing (nanopore
sequencing) |
References |
DNA amount |
Usually requires a higher quantity of DNA
(ranging from micrograms to milligrams),
depending on the specific method and
application. |
Requires a comparatively little
quantity of DNA,often ranging from
nanograms to micrograms. |
Without the need for an
amplification step. |
Gupta and Verma(2019),
Deamer et al. (2016) |
Quantification |
While correct quantification is vital,the
criteria are not as strict as for NGS. |
Accurate measurement is essential
because of the increased sensitivity
and decreased input demands. |
Quantitative PCR techniques
were used at Harvard to
quantify the number of DNA
molecules that passed to the
trans side of the pore. |
Kiehn and Car (2017),
Gupta and Verma(2019),
Deamer et al. (2016) |
Read Length |
Produces extended read lengths,frequently
reaching up to 1,000 bases or more. |
Typically produces shorter read
lengths than Sanger sequencing,
although modern technologies have
made some improvements. |
Up to 2.273 megabases or more. |
Nowrousian (2010),
Gupta and Verma(2019),
Wang et al. (2021) |
Cost |
Increased sequencing expenses. |
Reduced expense per sequenced. |
Higher costs,about $1,000. |
Gupta and Verma(2019),
Faulk(2023) |
Speed |
Lengthier procedure,particularly for large-
scale projects. |
Parallel processing capabilities result
in faster turnaround times,facilitating
rapid data creation. |
Less time required,about a few
hours. |
Gupta and Verma(2019),
Koch et al. (2023) |
Throughput |
The throughput is limited because to the
sequential processing of individual DNA
fragments. |
Utilizing high throughput technology,
this process allows for the
simultaneous sequencing of millions
of DNA fragments in parallel. |
Increased to~10-15 gigabases. |
Slatko et al. (2018),Wang
et al. (2021) |
Emerging Detection Technologies: Isothermal Amplification and CRISPR/Cas
Conventional polymerase chain reaction (PCR) and sequencing techniques, constrained by the necessity for high-temperature and thermocycling instruments, are predominantly relegated to laboratory analyses. Emerging isothermal amplification technologies demonstrate potential in overcoming these limitations. Unlike PCR, isothermal amplification obviates the need for thermal cycling through direct helix denaturation, primer binding, and extension mediated by specific proteins or enzymes, thereby allowing the reaction to proceed at a constant temperature. Since the early 21st century, these technologies have advanced rapidly, with notable examples such as recombinase polymerase amplification (RPA) and loop-mediated isothermal amplification (LAMP) being employed for the detection of methicillin-resistant Staphylococcus aureus in 2006 and 2007, respectively. Although the stability and specificity are inferior to those of conventional PCR, isothermal amplification offers rapid and sensitive detection (ranging from 10 to 60 minutes, capable of detecting single-digit copies per microliter), and hence, exhibits substantial developmental potential.
Similarly, the CRISPR/Cas system facilitates the detection of antibiotic resistance genes (ARGs) under low-temperature, isothermal conditions. As part of the bacterial adaptive immune system, CRISPR/Cas possesses significant gene-targeting and editing capabilities. This system identifies, integrates, and deactivates exogenous genes through the transcription of single-guide RNA sequences complementary to target sequences and encoding functional Cas proteins. Cas12 and Cas13 proteins are frequently applied in the development of nucleic acid detection technologies, where the incorporation of fluorescent probes into the reaction can enable quantitative detection of targets. Although CRISPR/Cas systems offer straightforward reaction conditions and rapid results (less than one hour), their limited sensitivity (ranging from 6×10^5 to 3×10^9 copies per microliter) challenges their standalone efficacy for target detection.
The realization of in situ rapid detection constitutes a predominant technical demand in contemporary ARG detection, necessitating breakthroughs in sensitivity and analytical speed on the foundation of extant methodologies. The integration of isothermal amplification with CRISPR/Cas systems presents a highly promising technological synergy, wherein target amplification ensures method sensitivity, and CRISPR/Cas-mediated target recognition ensures specificity. Documented research predominantly addresses viral detection, with notable examples including the SHERLOCK (Specific High-sensitivity Enzymatic Reporter unlocking) system based on LAMP and CRISPR/Cas13a, and the DETECTR (DNA Endonuclease-Targeted CRISPR Trans Reporter) system developed using RPA and CRISPR/Cas12a. Reports concerning ARG detection applications remain scarce, underscoring the necessity for continuous optimization and exploration for advancements in this domain.
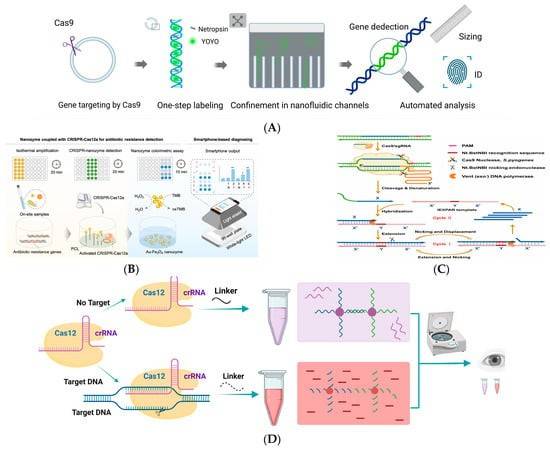 The CRISPR/Cas system-based detection platform for antibiotic-resistance genes. (Xuejiao Zhang et al,.2024)
The CRISPR/Cas system-based detection platform for antibiotic-resistance genes. (Xuejiao Zhang et al,.2024)
Other Fluorescence-Based Techniques
Other fluorescence-based strategies have also found application in the detection of ARGs. For instance, an improved fluorescence in situ hybridization technique, known as RING-FISH (Recognition of Individual Genes by Fluorescence In Situ Hybridization), has been employed to detect β-lactamase in Escherichia coli, achieving a sensitivity of <10 copies per cell.
DNA microarray technology, characterized by its rapid processing and high-throughput capabilities, has been utilized to detect 23 tetracycline-related ARGs and 10 erythromycin-related ARGs from soil and animal fecal samples.
Various types of fluorescence probes offer additional flexibility. These probes not only allow for the independent identification of targets and generation of signals, but can also be integrated with other techniques, such as PCR and isothermal amplification, to enhance detection performance.
Services you may interested in
Case Study:
Metagenomic Analysis Revealing the Occurrence of the Antibiotic Resistome in Salt Lakes
Journal: Science of the Total Environment
Publication Date: June 4, 2021
DOI: 10.1126/sciadv.abq8015
Overview
This study employed metagenomic approaches to investigate the broad-spectrum ARG profiles, microbial community composition, and the complex relationships between microbial communities and antibiotic resistance in four salt lakes. A total of 175 ARG subtypes and 19 ARG types were identified, with multidrug resistance, peptide antibiotics, and macrolide-lincosamide-streptogramin (MLS) resistance genes comprising 71.2% of the total ARG abundance. However, a significant decrease in ARG abundance was observed with increasing lake salinity. Notable differences were found in both the ARG profiles and microbial community structures across different lakes and sample types. Network analysis was conducted to further explore the relationships between ARGs and microbial assemblages, hypothesizing potential ARG hosts for MLS, multidrug, quinolone, peptide, fusidic acid, and rifamycin resistance genes. In conclusion, this study provides new insights into the occurrence and distribution of ARGs in hypersaline ecosystems.
Global Biogeography and Projection of Soil Antibiotic Resistance Genes
Journal: Science Advances
Publication Date: November 16, 2022
DOI: 10.1016/j.scitotenv.2021.148262
Overview
This study conducted a global analysis of 1,088 soil metagenomic samples, identifying 558 ARGs present in soil ecosystems. The abundance of ARGs in agricultural habitats was found to be significantly higher compared to non-agricultural habitats. The primary carriers of soil ARGs were clinical pathogens and intestinal microbiota, which mediate the influence of climate and anthropogenic factors on ARG prevalence.
Through the analysis of large-scale soil metagenomic datasets, the research team developed the first global distribution map of soil ARGs. This map revealed that hotspots of soil microbial resistance are predominantly located in densely populated and agriculturally developed regions, including the eastern United States, western Europe, South Asia, and East Asia.
Microbial annotations of gene sequences harboring ARGs indicated that these resistance genes are predominantly carried by intestinal microbiota and pathogenic microorganisms. Further analysis suggested that human activities, such as the use of sewage sludge and manure application, may introduce intestinal microbiota and pathogens into the soil, thereby increasing the abundance of soil ARGs. Additionally, geographic factors such as soil physicochemical properties, temperature, and precipitation were found to indirectly influence the proliferation of pathogens and intestinal microbiota, thus driving soil microbial resistance levels.
The findings of this study not only provide baseline information on soil ARGs at a global scale but also lay the groundwork for future modeling efforts in the context of climate change and anthropogenic scenarios.
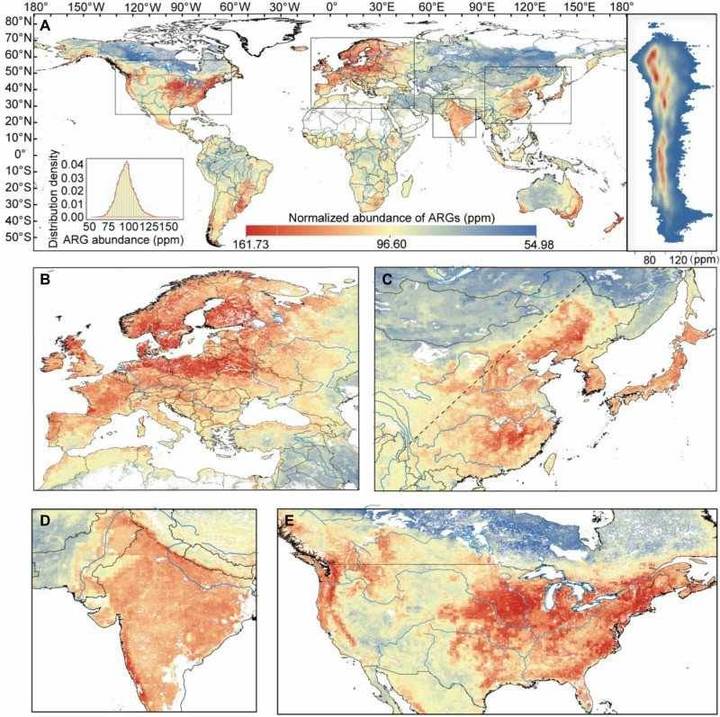 Global map of the normalized abundance of soil ARGs.
Global map of the normalized abundance of soil ARGs.
Microplastic Diversity Increases the Abundance of Antibiotic Resistance Genes in Soil
Journal: Nature Communications
Publication Date: November 12, 2024
DOI: https://doi.org/10.1038/s41467-024-54237-7
Overview
This study employed metagenomic analysis to investigate the impact of microplastic diversity on the dynamics of ARGs in soil ecosystems. Additionally, the potential health risks associated with the presence of virulence factor genes (VFGs) and mobile genetic elements (MGEs) were assessed. The findings revealed that an increase in microplastic diversity corresponded to higher abundances of ARGs, VFGs, and MGEs within the soil.
Further investigation highlighted that microplastic diversity may contribute to the spread of ARGs through microbial adaptation strategies that involve genetic diversity alterations, community-specific increases in particular microorganisms, and enriched metabolic pathways. These mechanisms facilitate the dissemination of resistance genes. The study also observed that other global change factors, such as the application of antimicrobial agents and reductions in plant biodiversity, were associated with elevated ARG levels in the soil.
The results underscore the importance of monitoring microplastic diversity within ecosystems, as it may promote the propagation of antibiotic resistance through multiple pathways. This provides new insights into the risks posed by the widespread presence of ARGs in terrestrial ecosystems.
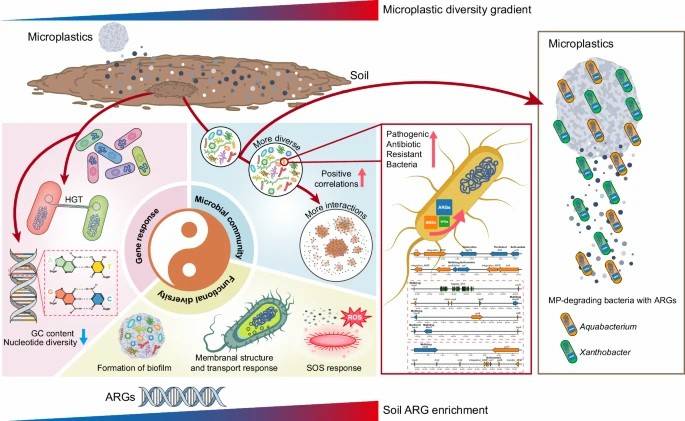 Conceptual model illustrating the multiple changes in the soil microbiome that contribute to antibiotic resistance enrichment in response to increasing MP diversity.
Conceptual model illustrating the multiple changes in the soil microbiome that contribute to antibiotic resistance enrichment in response to increasing MP diversity.
Summary and Outlook
Current commonly used methods for the detection of antibiotic resistance genes (ARGs) still possess significant potential for optimization and development. Both PCR and sequencing-based techniques are capable of fulfilling the demands for precise quantification and non-targeted screening. However, factors such as reliance on specialized instrumentation, time consumption, and economic cost limit the application of these methods in the rapid in situ detection of ARGs.
Isothermal amplification techniques, while capable of achieving sensitivity down to a single copy per microliter and reducing detection time to less than one hour, inherently sacrifice specificity, accuracy, and stability to some extent. The integration of CRISPR/Cas systems with isothermal amplification holds the greatest promise for overcoming these technological limitations. However, the widespread practical application of this combined approach requires further optimization in laboratory settings and the subsequent development of commercial solutions. This remains a key area of focus for future research and technological advancement within the field.


 Detecting antibiotic genes in natural samples.a A PCR-based approach. b Functional metagenomics. (Heather K Allen et al,.2010)
Detecting antibiotic genes in natural samples.a A PCR-based approach. b Functional metagenomics. (Heather K Allen et al,.2010) The CRISPR/Cas system-based detection platform for antibiotic-resistance genes. (Xuejiao Zhang et al,.2024)
The CRISPR/Cas system-based detection platform for antibiotic-resistance genes. (Xuejiao Zhang et al,.2024) Global map of the normalized abundance of soil ARGs.
Global map of the normalized abundance of soil ARGs. Conceptual model illustrating the multiple changes in the soil microbiome that contribute to antibiotic resistance enrichment in response to increasing MP diversity.
Conceptual model illustrating the multiple changes in the soil microbiome that contribute to antibiotic resistance enrichment in response to increasing MP diversity.