Antibiotics: Definition, Origin, and Types
Definition of Antibiotics
Antibiotics occupy a pivotal role in modern medicine as substances derived from the secondary metabolites of microorganisms, higher plants, and animals during their life activities. These compounds, along with their synthetic and semi-synthetic analogs, possess potent antimicrobial or other physiological activities. In a simplified analogy, antibiotics function as precise "surgical tools" that disrupt and modulate the development and functioning of other cells. Rather than acting directly upon human cells, antibiotics target invading pathogens such as bacteria and fungi. By inhibiting or eliminating these pathogens, antibiotics alleviate the burden on the human immune system, thus facilitating the treatment of diseases. Their unique mechanisms of action and physiological activity render them indispensable weapons in the fight against infectious diseases in contemporary medical practice. This paper systematically analyzes the fundamental characteristics of antibiotics, elucidates their modes of action and mechanisms of resistance development, tracks the transmission pathways of resistance genes, and explores multidimensional strategies to address these challenges, aiming to provide a scientific basis for curbing the antibiotic resistance crisis.
Sources of Antibiotics
Antibiotic sources are varied, primarily divided into naturally derived and synthetically produced categories. Within the domain of natural sources, microbial fermentation is of paramount importance. Several microorganisms inherently generate antibiotics as part of their metabolic processes. For example, species of the genus Penicillium are responsible for the production of penicillin, whereas species of the genus Streptomyces produce streptomycin. These naturally occurring antibiotics have led to revolutionary advancements in the medical sciences.
Advancements in technology have facilitated the development of synthetic and semi-synthetic antibiotics. Synthetic antibiotics are synthesized entirely through chemical methods within laboratory environments. Conversely, semi-synthetic antibiotics are produced by chemically altering the structure of natural antibiotics. Amoxicillin serves as an illustrative example of a semi-synthetic antibiotic, achieved through the structural modification of penicillin. This alteration not only maintains its antimicrobial efficacy but also enhances its pharmacokinetic properties, thus extending its range of clinical applications.
Types of Antibiotics
There exists a diverse array of antibiotic types, each characterized by distinct properties and specific applications. The β-lactam antibiotics, which include well-known agents such as penicillins and cephalosporins, constitute a prominent category frequently utilized in clinical practice. These antibiotics target the bacterial cell wall by binding to penicillin-binding proteins, thereby inhibiting cell wall synthesis and effecting bactericidal activity. Their broad antimicrobial spectrum renders them effective against a wide range of Gram-positive and Gram-negative bacteria, making them commonly prescribed for infections of the respiratory tract, skin, and soft tissues.
Aminoglycoside antibiotics, such as gentamicin and streptomycin, exert their effects primarily by binding to the 30S subunit of the bacterial ribosome, thus inhibiting protein synthesis. They exhibit potent antibacterial activity against aerobic Gram-negative bacilli and are often reserved for the treatment of severe infections caused by these organisms.
Macrolide antibiotics, including erythromycin and azithromycin, act on the 50S subunit of the bacterial ribosome, also inhibiting protein synthesis. Their antimicrobial spectrum is similar to that of penicillins, with notable efficacy against atypical pathogens such as Mycoplasma and Chlamydia spp. Consequently, they are frequently used in the management of respiratory tract and urogenital infections.
The diversity of antibiotic classes available in clinical practice offers a wide range of therapeutic options, enabling clinicians to address the various complexities encountered in infectious disease management.
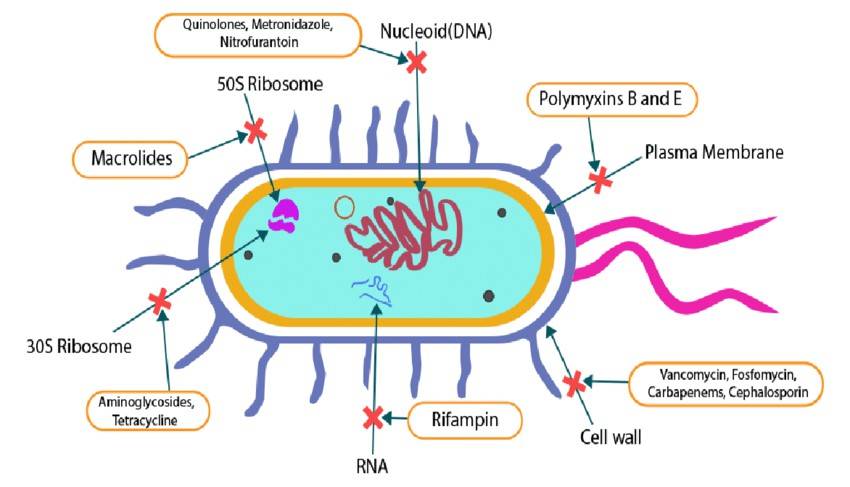 Sites of action for different types of antibiotics.(Sartini Natsir et al,.2021)
Sites of action for different types of antibiotics.(Sartini Natsir et al,.2021)
Mechanism of Action of Antibiotics
Inhibition of Bacterial Cell Wall Synthesis
The bacterial cell wall functions as a robust protective barrier, quintessential for maintaining cell morphology, facilitating substance transport, and stabilizing osmotic pressure. β-lactam antibiotics function analogously to precise "disruptors," targeting penicillin-binding proteins (PBPs) located on the bacterial cell wall. Upon binding tightly to PBPs, β-lactam antibiotics effectively obstruct the normal activity of transpeptidases, key enzymes responsible for the critical cross-linking of peptidoglycans during cell wall synthesis. This is comparable to the absence of essential connecting components in house construction, leading to an unstable structure.
With the interruption of cell wall synthesis, the integrity of the bacterial cell wall becomes compromised, rendering it incapable of withstanding the osmotic pressure differential between the intracellular and extracellular environments. Consequently, external water rushes into the bacterial cell analogous to a surging tide, causing rapid swelling and deformation of the cell. Ultimately, this results in the cell bursting and dying, akin to an overinflated balloon, thereby effectively inhibiting bacterial growth and proliferation.
Alteration of Bacterial Cell Membrane Permeability
Certain antibiotics function as "disruptors" of the bacterial cell membrane, interacting specifically with the membrane components to alter its permeability. Polypeptide antibiotics, such as polymyxin E, exemplify this mode of action. Possessing cationic properties, these antibiotics resemble positively charged "claws" that can tightly grasp phospholipid molecules on the bacterial cell membrane. This interaction disrupts the ordered structure of the membrane, compromising its integrity as if creating small "tears" within the membrane itself.
As a consequence, essential intracellular substances, including salts critical for maintaining normal cellular function, proteins involved in various metabolic reactions, nucleic acids carrying genetic information, and amino acids constituting proteins, are akin to a "treasure" leaking through these created "breaches." The continuous efflux of these vital components results in an inability of the bacterial cell to sustain normal physiological functions, ultimately leading to cell death. However, due to structural similarities between bacterial and human cell membranes, the application of such antibiotics could pose potential health risks to human cells.
Inhibition of Bacterial Protein Synthesis
Protein synthesis is pivotal for the survival and proliferation of bacteria, with the bacterial ribosome acting as the central "factory" in this process. Notably, there exist significant structural differences between bacterial and human ribosomes. Bacterial ribosomes are characterized as 70S complexes composed of 50S and 30S subunits, whereas human ribosomes are 80S complexes comprising 60S and 40S subunits. This structural disparity provides a "strategic opportunity" for antibiotics to exert their effects selectively on bacteria.
Various antibiotics function as precise "interferers," targeting different subunits of the bacterial ribosome. Aminoglycosides and tetracyclines specifically target the 30S subunit, akin to creating obstacles in the "raw material supply line" of the factory, thereby interfering with the proper interaction between mRNA and ribosomes and inhibiting the initiation phase of protein synthesis.
Conversely, chloramphenicol, macrolides, and lincosamides focus on the 50S subunit, comparable to disrupting critical pathways in the "production line," thereby hindering peptide chain elongation and the termination processes of protein synthesis. Through these mechanisms, antibiotics effectively inhibit bacterial protein synthesis, preventing the bacteria from producing essential proteins necessary for their survival and metabolic activities, thus achieving the goal of bacterial growth inhibition.
Inhibition of Bacterial Nucleic Acid and Folic Acid Metabolism
The metabolism of nucleic acids and folic acid in bacteria serves as the "engine" driving their vital activities, playing a key role in bacterial growth and reproduction. Sulfonamide drugs act as "mimics" due to their structural similarity to para-aminobenzoic acid (PABA). During bacterial metabolism, sulfonamides "disguise" as PABA and competitively bind to dihydropteroate synthase. This scenario is akin to an "imposter" overtaking the rightful competitor's place in a contest, preventing dihydropteroate synthase from catalyzing the conversion of PABA to dihydropteroic acid, thereby disrupting the synthesis of folic acid within the bacterial cell. Since folic acid is essential for nucleotide synthesis in bacteria, its impaired synthesis leads to restricted nucleotide production, consequently inhibiting bacterial growth and proliferation.
Conversely, quinolone antibiotics function as "genetic disruptors," selectively binding to bacterial DNA or RNA and directly interfering with the DNA replication process. This is akin to erecting multiple barriers on the "replication assembly line" of DNA, obstructing its progression. Furthermore, quinolones may induce direct damage to the DNA strand, akin to severing critical links in the "chain" of DNA, severely impacting the transmission and expression of bacterial genetic information. This disruption effectively curbs bacterial growth and reproduction.
Antibiotic Resistance: Definition, Impact, and Mechanisms
Definition of Antibiotic Resistance
Antibiotic resistance is a formidable and intricate phenomenon whereby microorganisms, including bacteria and fungi, progressively develop a suite of resistance mechanisms through prolonged exposure to antibiotics. This evolution renders antibiotics, which were previously effective in inhibiting or eradicating these organisms, ineffectual. The microorganisms essentially evolve a "defense mechanism" that impedes the penetration and efficacy of antibiotics. Under appropriate dosage and treatment regimens, antibiotics can typically target specific microbial processes, disrupting their normal physiological functions to control infections. However, with the advent of resistance, microorganisms employ strategies such as altering their structural attributes, producing specific enzymes, or modifying metabolic pathways to evade the effects of antibiotics. This enables them to persist and proliferate within the host, complicating the control of infections and transforming once-treatable diseases into challenging clinical hurdles.
Impact of Antibiotic Resistance
Antibiotic resistance poses a significant challenge across healthcare, agriculture, and ecosystem domains. In the medical sector, this resistance directly results in therapeutic failures. In the past, simple bacterial infections could be effectively treated with standard antibiotics. However, the emergence of resistant strains has now rendered these treatments prolonged and challenging. Patients may require advanced, more costly antibiotics or even combination therapies, which not only increase healthcare expenditures but also elevate the risk of adverse drug reactions. Additionally, the risk of disease transmission is substantially heightened. Resistant bacteria persist longer in patients, facilitating their spread, particularly in densely populated settings such as hospitals, potentially leading to widespread outbreaks of resistant infections.
In the agricultural sector, the extensive use of antibiotics for the prevention and treatment of animal diseases has fostered the proliferation of resistant bacterial strains within livestock. These resistant bacteria adversely affect animal health, diminishing the efficiency of agricultural production, and can be transmitted through the food chain to humans, increasing the risk of human infections with resistant strains.
From an ecological perspective, residual antibiotics in the environment promote the development of resistance in microorganisms, disrupting microbial community balance. This can impair ecosystem functions such as nutrient cycling and energy flow, ultimately threatening the stability of the entire ecosystem.
Mechanisms Underlying the Development of Antibiotic Resistance
The emergence of antibiotic resistance is a multifaceted phenomenon driven by various factors. Primarily, the misuse and overuse of antibiotics play a pivotal role. In medical settings, some healthcare professionals prescribe antibiotics inappropriately, while patients often do not adhere to prescribed treatment regimens. This non-compliance includes arbitrary changes in dosage or premature cessation of treatment, resulting in incomplete eradication of bacteria. Consequently, bacteria are exposed to suboptimal concentrations of antibiotics, leading to the selection and evolution of resistant strains. In the agricultural sector, the quest for higher yields often involves the excessive inclusion of antibiotics in animal feed, creating an environment conducive to the development of resistance among bacteria within the animals.
Furthermore, genetic mutations within microorganisms also contribute significantly to resistance. During microbial reproduction, errors in DNA replication can result in mutations, some of which may confer antibiotic resistance. Under selective antibiotic pressure, resistant mutant strains are preferentially selected and can proliferate extensively.
Horizontal gene transfer constitutes another major pathway for the dissemination of resistance. Bacteria are capable of exchanging genetic material through mechanisms such as transformation, transduction, and conjugation. This gene transfer allows even initially susceptible bacteria to acquire resistance genes, thereby transforming them into resistant strains and exacerbating the spread of resistance within bacterial populations.
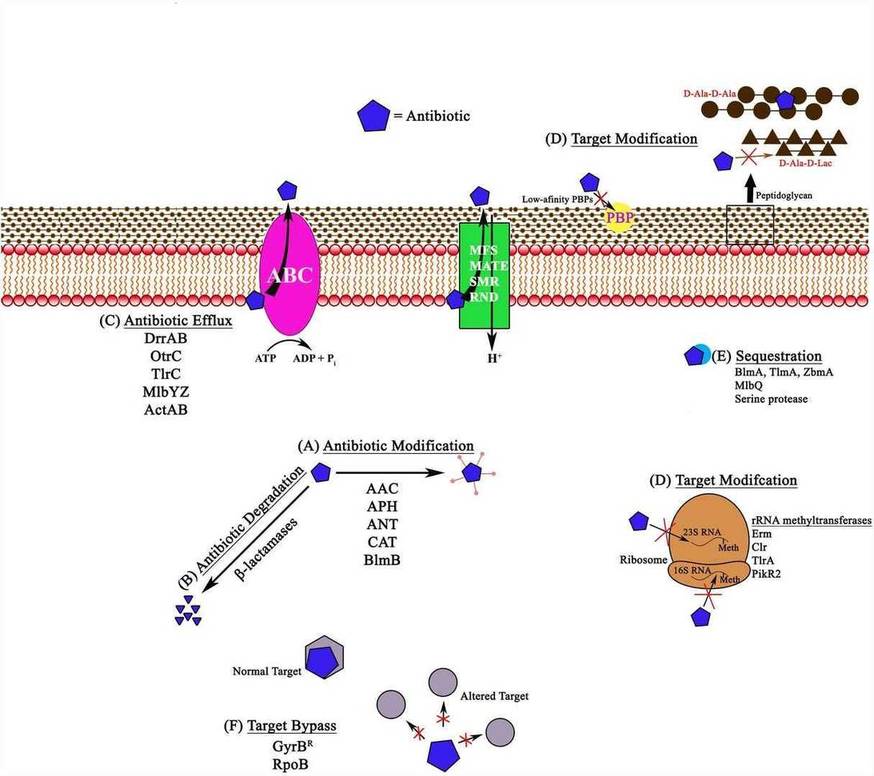 Schematic representation of different antibiotic resistance mechanisms in bacteria, shown with examples. (Elizabeth Peterson et al,.2018)
Schematic representation of different antibiotic resistance mechanisms in bacteria, shown with examples. (Elizabeth Peterson et al,.2018)
Production of Enzymes for Antibiotic Inactivation or Modification
To withstand the effects of antibiotics, bacteria have evolved the capacity to produce enzymes that inactivate or modify these drugs, serving as a principal mechanism of antibiotic resistance. The production of β-lactamases represents a primary example of such enzymatic defense mechanisms. These enzymes function by precisely cleaving the β-lactam ring, a critical structural feature of β-lactam antibiotics. Once this essential configuration is disrupted, the antibiotic loses its antimicrobial efficacy and can no longer effectively inhibit bacterial cell wall synthesis.
In addition to β-lactamases, aminoglycoside-modifying enzymes also play a significant role in bacterial resistance. These enzymes chemomodify aminoglycoside antibiotics, altering their chemical structure. This modification prevents the antibiotics from binding appropriately to the 30S subunit of bacterial ribosomes, thus incapacitating their ability to interfere with bacterial protein synthesis. Consequently, bacteria effectively evade the inhibitory action of these antibiotics.
The production of such inactivating or modifying enzymes is a vital survival strategy that enables bacteria to persist in the presence of antibiotics, ultimately contributing to the persistence of resistant bacterial populations under antibiotic pressure.
Alteration of Drug Target Sites
Bacteria have developed strategies to diminish antibiotic binding affinity and efficacy by altering drug target sites. One such strategy involves structural modifications of the target. Bacterial proteins, which serve as specific binding sites for certain antibiotics, can undergo conformational changes through mechanisms such as genetic mutations. These alterations disrupt the "lock-and-key" fit between the antibiotic and its target, thereby hampering the antibiotic's ability to bind effectively and exert its therapeutic action.
Additionally, bacteria can modulate the number or expression levels of these targets. In response to the perceived threat of antibiotic presence, bacteria may increase the number of target molecules, thereby diluting the impact of the antibiotic, as the available drug molecules become insufficient to interact with all targets. Conversely, bacteria may downregulate the expression of these targets, reducing the number of binding sites available for the antibiotic. Both strategies collectively weaken the overall effectiveness of the antibiotic, contributing to resistance.
These adaptive mechanisms enable bacteria to sustain growth and survival even under the selective pressure of antibiotic treatment, facilitating the persistence and spread of antibiotic-resistant strains.
Modification of Cell Membrane Permeability
Bacteria employ strategies to alter cell membrane structures or reduce the presence of membrane-associated porin proteins, effectively constructing a barrier that impedes antibiotic entry. These porin proteins function as "gates" on the cellular membrane, facilitating the passage of antibiotics into the bacterial cell under normal circumstances. However, in an attempt to counteract antibiotic effects, bacteria can modify the structure of the cell membrane, leading to alterations in the shape or functionality of these "gates."
Additionally, bacteria may decrease the abundance of these porin proteins or eliminate specific porin channels altogether. This is akin to closing certain "gates" or rendering them challenging to recognize and traverse. Consequently, the difficulty for antibiotics to penetrate the cell and reach their intended intracellular targets is significantly heightened. This impaired antibiotic entry reduces the bactericidal effectiveness of the drugs, allowing bacteria to persist and thrive even in antibiotic-rich environments.
Such adaptive changes in membrane permeability contribute to bacterial survival by severely limiting the access of antibiotics to their action sites within the bacterial cell, thereby facilitating the emergence and maintenance of antibiotic resistance.
Efflux Pump Mechanism
Bacterial efflux pumps function analogously to intracellular "pumps," actively exporting antibiotics from the cell and thereby reducing intracellular drug concentrations. These efflux pumps are typically sophisticated systems composed of multiple proteins, characterized by their energy dependence. They utilize cellular energy, such as that derived from ATP hydrolysis, to expel antibiotics against their concentration gradient, out of the cell.
Upon antibiotic entry into bacterial cells, efflux pumps are rapidly activated, continuously removing the antibiotic molecules from the intracellular environment. This action ensures that the concentration of antibiotics within the cell remains consistently low, preventing the drugs from reaching the threshold needed to effectively inhibit or kill the bacteria.
The efflux pump mechanism is prevalent across diverse bacterial species, representing a critical self-protective strategy. This mechanism enables bacteria to survive and proliferate even under the selective pressure exerted by antibiotic treatments, thereby contributing significantly to antibiotic resistance.
Biofilm Formation
The formation of bacterial biofilms is a complex and intricate process. Initially, individual bacterial cells attach to a surface, subsequently secreting extracellular polysaccharides, proteins, and other substances, gradually developing a sticky matrix that encapsulates numerous bacterial cells and culminates in the establishment of a biofilm. For bacteria, the biofilm functions as a robust "fortress," fulfilling several critical roles. It provides physical protection, shielding bacteria from adverse environmental conditions, and also impedes the penetration and efficacy of antibiotics.
As antibiotics attempt to infiltrate the biofilm, they encounter substantial barriers. The viscous matrix of the biofilm acts akin to a mesh filter, trapping antibiotics and preventing them from reaching the bacteria encased within. Furthermore, bacterial cells within the biofilm exhibit reduced metabolic activity, leading to decreased sensitivity to antibiotics. This further complicates the ability of antibiotics to exert their therapeutic effects.
The biofilm's protective mechanisms significantly contribute to bacterial survival and resistance, presenting a formidable challenge in the treatment of biofilm-associated infections.
Cross-Resistance
Cross-resistance represents a specialized phenomenon of bacterial resistance wherein a bacterium that has developed resistance to one antibiotic may also exhibit resistance to other antibiotics with similar mechanisms of action. This occurrence is attributable to the fact that antibiotics with analogous mechanisms typically target the same or related physiological processes or sites within the bacterial cell.
When bacteria acquire resistance to a particular antibiotic, for example, by altering the structure of a target site so that it no longer binds effectively to the antibiotic, other antibiotics targeting the same site or similar physiological pathways may also become ineffective due to the same modifications.
This phenomenon of cross-resistance broadens the scope of bacterial defenses against antibiotics, potentially rendering multiple previously effective antibiotics ineffective due to resistance to a single agent. Consequently, cross-resistance poses significant challenges to clinical treatment, complicating efforts to manage and control bacterial infections and necessitating the development of new therapeutic strategies.
Alteration of Target Structures
Changes in bacterial target structures profoundly affect the binding affinity between antibiotics and their intended targets, leading to resistance. The interaction between an antibiotic and its target is analogous to a precise "molecular lock-and-key" fit, necessitating specific structural complementarity and interactions.
When bacterial target structures undergo modifications, this precise matching relationship is disrupted. For instance, alterations in the amino acid sequence of a target protein can lead to changes in its spatial configuration, rendering previously compatible binding sites misaligned. This scenario is akin to a key that once fit perfectly into a lock but can no longer enter or turn after the lock's internal structure changes.
The reduced binding affinity between the antibiotic and the altered target diminishes the antibiotic's capacity to bind effectively and exert its intended action. Consequently, bacteria acquire resistance to the antibiotic, allowing them to continue to survive and proliferate within the host. This mechanism underscores the significance of structural alterations in bacterial resistance strategies.
Antibiotic Resistance Genes: Definition, Origin, Impact, and Mechanisms of Dissemination
Definition and Origin of Antibiotic Resistance Genes
Antibiotic resistance genes (ARGs) are specialized genetic elements embedded within the genomes of bacteria or other microorganisms, akin to "mysterious codes" that endow microbes with the capability to withstand antibiotic attack. These genes encode various proteins or bioactive molecules that enable microorganisms to survive and propagate in environments containing antibiotics. The origin of ARGs is a complex and lengthy process, with natural evolution being a significant contributor. Over extensive evolutionary timescales, microorganisms undergo spontaneous genetic mutations to adapt to the ever-changing environment. Some of these mutations yield genes that confer a degree of resistance, a result of natural selection.
However, the widespread and, notably, the irrational use of modern antibiotics has exerted substantial selective pressure. The extensive application of antibiotics has facilitated the rapid selection and enrichment of resistance genes within microbial populations, which were otherwise present at low levels. This accelerated dissemination and proliferation of ARGs have exacerbated the antibiotic resistance problem, highlighting the urgent need for more prudent antibiotic utilization.
Services you may interested in
Impact of Antibiotic Resistance Genes
ARGs pose a substantial threat to human health, agricultural productivity, and ecological balance. In the realm of human health, the presence of ARGs severely complicates the management and treatment of bacterial infections. The reduced efficacy of conventional antibiotics can render previously easily curable diseases persistent, leading to prolonged patient suffering, extended hospital stays, and significantly increased healthcare costs. More critically, infections with antibiotic-resistant bacteria can be life-threatening, contributing to higher mortality rates.
In the agricultural context, the presence of ARGs within livestock not only jeopardizes animal health and reduces production efficiency but also presents a risk of transmission to humans through the food chain, elevating the incidence of human infections with resistant strains. Moreover, the dissemination of ARGs within agricultural environments can alter soil microbial community structure and function, potentially impacting crop growth and soil fertility.
Within ecosystems, ARGs disrupt the natural balance of microbial communities. The selective advantage conferred to resistant bacteria may alter interspecies relationships, affecting nutrient cycling and energy flow within ecosystems. The resultant destabilization can lead to unforeseen consequences on the overall stability and functionality of ecological systems.
Mechanisms of Antibiotic Resistance Gene Dissemination
The dissemination of ARGs occurs through diverse mechanisms, primarily characterized by vertical and horizontal transmission. Vertical transmission involves the transfer of resistance genes from parent microorganisms to their offspring, akin to passing a baton in a relay race. During microbial reproduction, parental bacteria pass their ARGs intact to progeny, perpetuating and spreading resistance traits within bacterial populations.
Horizontal transmission, however, is more complex and poses a greater threat by transcending species boundaries. One mode of horizontal transmission is transformation, wherein bacteria uptake free-floating DNA fragments from the surrounding environment. If these fragments contain ARGs, the bacteria may integrate them into their genome, thereby acquiring resistance capabilities. Another mode, transduction, involves bacteriophages-viruses that infect bacteria-which can transfer ARGs into bacterial cells during the infection process, converting previously susceptible bacteria into resistant strains.
Conjugation represents yet another mechanism, where genetic material is exchanged between bacteria through direct contact. Utilizing specialized structures such as sex pili, resistant bacteria can transfer plasmids containing ARGs to other bacteria. These intertwined mechanisms collectively expedite the spread of ARGs across various microbial populations and environments, complicating efforts to manage and control antibiotic resistance.
Strategies to Combat Antibiotic Resistance
1. Curbing Antibiotic Misuse
Addressing antibiotic misuse is a fundamental step in tackling resistance issues, necessitating robust measures across both medical and agricultural sectors. In the healthcare field, developing comprehensive guidelines for antibiotic use is crucial. Professional medical organizations and institutions should base these guidelines on clinical research and empirical experience, clearly delineating the appropriate indications, dosages, and durations of antibiotic therapy. This provides physicians with explicit guidance for antibiotic prescriptions. Additionally, education and training programs for healthcare providers should be enhanced to improve their understanding and competence in the rational use of antibiotics, thereby reducing unnecessary prescriptions. Strengthening patient education is also vital to ensure they understand proper antibiotic usage, avoiding self-medication and unsanctioned treatment interruption. On a regulatory level, implementing stringent prescription review systems can effectively intervene and correct inappropriate antibiotic use promptly.
In the agricultural sector, efforts to reduce the use of antibiotics as growth promoters in animal feed must be prioritized. Promoting sustainable farming practices-such as improving farming conditions and optimizing feed formulations-can enhance animal immunity and reduce disease incidence. Governmental agencies should enforce stricter regulations on the livestock industry and increase penalties for non-compliance with antibiotic policies, ensuring effective implementation of these policies. By implementing these strategies, the irrational use of antibiotics can be significantly diminished, thereby reducing the emergence and spread of resistant bacteria.
Technological Innovations
Technological innovations offer new avenues and hope in the fight against antibiotic resistance. The advancement of rapid diagnostic technologies facilitates the prompt detection of resistance genes. For example, progress in gene sequencing technologies, particularly the application of the CRISPR-Cas system, enables the precise identification of resistance genes harbored by bacteria in a short timeframe. This capability assists clinicians in selecting the most effective antibiotics at the outset of treatment, avoiding empirical therapy, reducing unnecessary antibiotic exposure, and thereby diminishing the emergence of resistance.
Novel therapeutic approaches are also continually emerging. Phage therapy, an age-old yet increasingly relevant treatment strategy, is regaining attention. Bacteriophages, viruses that specifically infect bacteria, offer a high degree of specificity, targeting and eliminating resistant bacteria without readily inducing new resistance. In the United States, phage therapy has been successfully employed to treat patients with infections caused by multidrug-resistant pathogens, presenting a new clinical treatment option.
Moreover, gene editing technologies such as CRISPR hold the potential for eradicating resistance genes. By precisely editing bacterial genomes, it is possible to specifically delete or modify resistance genes, potentially restoring bacterial susceptibility to antibiotics. In the realm of drug development, inhibitors targeting bacterial efflux pumps and biofilms are under active investigation and clinical trials. These innovative technologies and pharmacological advancements promise robust support in addressing the antibiotic resistance challenge.
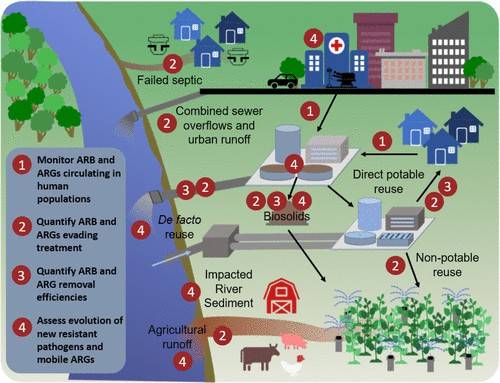 Monitoring objectives and transmission pathways for antimicrobials, resistant microorganisms, mobile genetic elements (MGEs), and antibiotic resistance genes (ARGs) in humans and the environment. (Krista Liguori et al,.2022)
Monitoring objectives and transmission pathways for antimicrobials, resistant microorganisms, mobile genetic elements (MGEs), and antibiotic resistance genes (ARGs) in humans and the environment. (Krista Liguori et al,.2022)
International Collaboration and Policy Formulation
Antibiotic resistance represents a global public health challenge, necessitating collaborative efforts among nations to effectively combat it. International organizations play a crucial role in coordinating these global actions. The World Health Organization (WHO), for instance, actively encourages countries to develop national action plans, enhance surveillance and research, and raise public awareness. By establishing a global surveillance network, WHO facilitates real-time tracking of the distribution and dissemination of resistant bacteria, providing the scientific basis for formulating targeted control strategies.
Countries globally are adopting proactive measures. Many have implemented stringent regulations to control antibiotic sales and usage. For example, Thailand has enacted laws to restrict over-the-counter sales of antibiotics, significantly reducing the incidence of resistant infections. The European Union has comprehensively banned the use of antibiotics as growth promoters in animal feed, addressing the overuse of antibiotics at its source. Additionally, nations are bolstering cooperation in the scientific research arena, sharing research findings and technical expertise to expedite the development of novel antibiotics and therapeutic approaches.
International collaboration and strategic policy formulation are critical in addressing the issue of antibiotic resistance. It is only through concerted global efforts that the spread of resistance can be effectively curtailed, safeguarding human health and ecological security.
References
- Peterson E, Kaur P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front Microbiol. 2018 Nov 30;9:2928. DOI: 10.3389/fmicb.2018.02928. PMID: 30555448; PMCID: PMC6283892.
- Uluseker C, Kaster KM, Thorsen K, Basiry D, Shobana S, Jain M, Kumar G, Kommedal R, Pala-Ozkok I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front Microbiol. 2021 Oct 11;12:717809. DOI: 10.3389/fmicb.2021.717809. PMID: 34707579; PMCID: PMC8542863.
- Krista Liguori, Ishi Keenum, Benjamin C. Davis, Jeanette Calarco, Erin Milligan, Valerie J. Harwood, and Amy Pruden Environmental Science & Technology 2022 56 (13), 9149-9160 https://doi.org/10.1021/acs.est.1c08918

 Sites of action for different types of antibiotics.(Sartini Natsir et al,.2021)
Sites of action for different types of antibiotics.(Sartini Natsir et al,.2021) Schematic representation of different antibiotic resistance mechanisms in bacteria, shown with examples. (Elizabeth Peterson et al,.2018)
Schematic representation of different antibiotic resistance mechanisms in bacteria, shown with examples. (Elizabeth Peterson et al,.2018) Monitoring objectives and transmission pathways for antimicrobials, resistant microorganisms, mobile genetic elements (MGEs), and antibiotic resistance genes (ARGs) in humans and the environment. (Krista Liguori et al,.2022)
Monitoring objectives and transmission pathways for antimicrobials, resistant microorganisms, mobile genetic elements (MGEs), and antibiotic resistance genes (ARGs) in humans and the environment. (Krista Liguori et al,.2022)