We use cookies to understand how you use our site and to improve the overall user experience. This includes personalizing content and advertising. Read our Privacy Policy
Genomics is currently undergoing a paradigm shift from a linear sequence to a three-dimensional space, with Hi-C technology serving as the primary catalyst for this transformation. This technological advancement is profoundly impacting our understanding of biological complexity. Conventional genomics emphasizes the interpretation of "alphabetical sequences." However, this approach falls short in elucidating critical issues such as non-coding region variation, remote gene regulation, and cellular heterogeneity. The advent of Hi-C technology has marked a paradigm shift in our understanding of genomes, transforming them from a one-dimensional "code" to a three-dimensional "dynamic network" through the capture of chromosome spatial interactions. This technological breakthrough has unveiled the three-dimensional regulation logic of gene expression, elucidating the mechanisms of disease and developmental programs. The advent of single-cell technology, multi-omics integration, and novel sequencing platforms has catapulted the application of Hi-C from the domain of fundamental research to the vanguard of clinical translation and precision medicine. In this paper, we will initiate our discussion with the foundational framework of 3D genomics. We will then proceed to systematically examine the existing applications, innovative breakthroughs, and future potential of Hi-C technology. Finally, we will explore how Hi-C technology can propel genomics from "structural analysis" to "functional intervention" in the new era.
Genomics research has evolved from the resolution of linear sequences to the exploration of three-dimensional spatial structures, a shift that has profoundly impacted our understanding of gene regulation and cellular function. Three-dimensional genomics has not only revealed the intricate folding patterns of DNA in the nucleus, but also elucidated the dynamic association between gene expression and spatial conformation. Hi-C technology is a central tool in this field, providing an unprecedented means of mapping the genome in 3D by capturing the physical interaction information between chromosomes. In the following discussion, we will start from the basic framework of 3D genomics and the principles of Hi-C technology and analyze step by step how it can reshape our perception of chromosome architecture.
Overview of 3D genomics and Hi-C technology
The advent of technology has precipitated a meteoric rise in the field of genomics. However, when contemplating the sequence structure of genes, the double helix configuration of DNA frequently springs to mind. In reality, genomic DNA manifests in a multifaceted arrangement within the cell nucleus, where these intricate layers sequentially condense the 2-meter-long DNA sequence into the 10-micron nucleus. The three-dimensional spatial configuration of this compressed structure facilitates the spatial proximity of gene regions that are distant in linear distance. Gene expression is modulated by regulatory elements in non-coding regions, in addition to being determined by their own coding sequences. Gene expression, regulation, and interactions between regulatory elements are achieved in the three-dimensional structure formed by chromosome folding. Consequently, the three-dimensional structure of the genome must be studied to reveal gene expression and regulation.
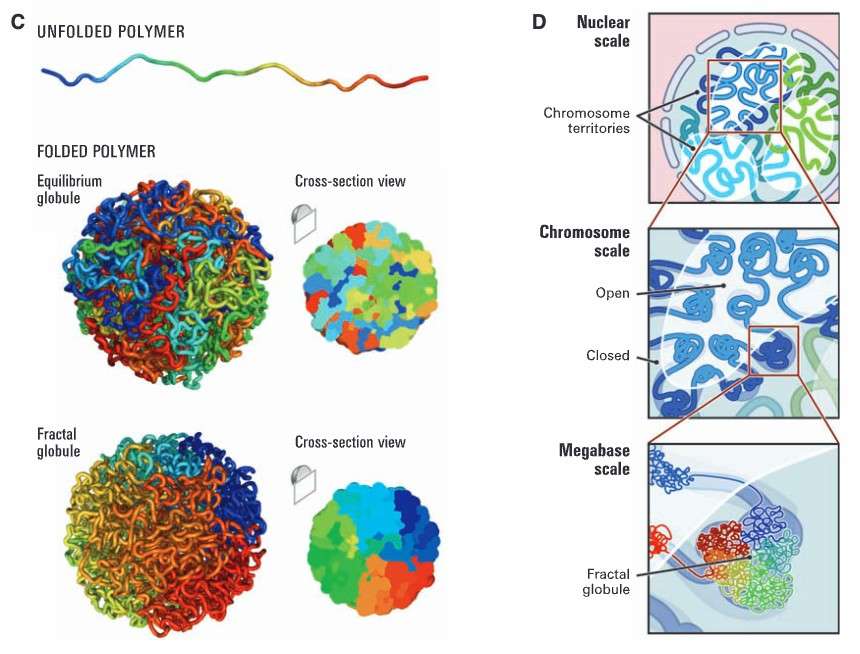 Genome architecture at threescales (Lieberman-Aiden et al., 2009)
Genome architecture at threescales (Lieberman-Aiden et al., 2009)
Organisms are capable of existing without altering the linear sequence of deoxyribonucleic acid (DNA) during periods of environmental change, developmental processes, and so forth. The 3D spatial structure of DNA is subject to regulation, thereby altering the spatial interactions between regulatory elements and target genes. This, in turn, regulates gene expression. Advancements in technology and in-depth research have led to a growing body of studies that demonstrate the significant role that 3D genome structure plays in regulating gene expression and cell function.
The study of the three-dimensional genome is primarily divided into four levels: chromatin frontiers, chromatin compartments, topologically associated structural domains, and chromatin loops. The most advanced tool for three-dimensional genome research is Hi-C sequencing technology, a new technology developed based on genome capture that combines high-throughput sequencing with a method to analyze the three-dimensional spatial structure of chromosomes and the genome (high-throughput chromosome conformation capture technology). This technology facilitates the detection of all unknown interaction regions within the entire genome, enabling the acquisition of high-resolution maps depicting chromatin regulatory element interactions. Consequently, it unveils remote interactions between chromosomes, the 3D spatial structure of the genome, and the specific regulatory relationships between associated genes.
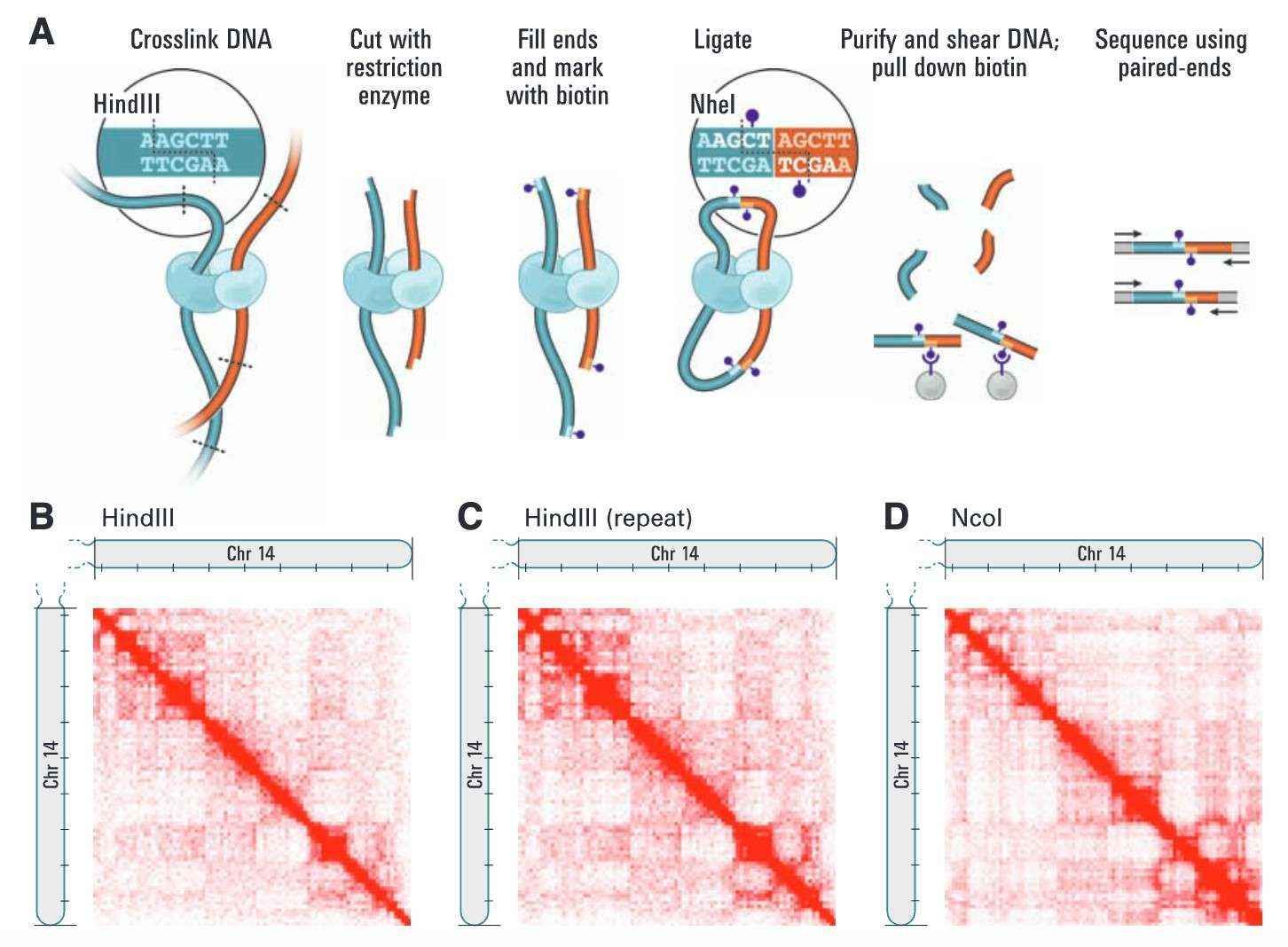 Overview of Hi-C (Lieberman-Aiden et al., 2009)
Overview of Hi-C (Lieberman-Aiden et al., 2009)
In 2009, Job Dekker's team published a seminal paper in Science entitled "Comprehensive mapping of long range interactions reveals folding principles of the human genome." This paper was the first to propose the Hi-C technique and analyze the spatial interaction information of the loci in the chromosomes of human normal lymphoblastoid cells. Since then, a substantial body of literature has emerged exploring the three-dimensional structure of chromosomes using Hi-C. This seminal paper introduced the Hi-C technique, a pioneering approach that analyzed the spatial interaction information of loci in the chromosomes of normal human lymphoblastoid cells. Subsequent studies have increasingly employed the Hi-C technique to study the 3D structure of chromosomes. The Hi-C technology is a relatively straightforward process that integrates chromatin conformation capture with high-throughput sequencing. The primary technical procedure is subdivided into six stages: chromatin cross-linking, endonuclease digestion, biotin leveling, ligation, DNA purification and capture, and high-throughput sequencing.
The significance of Hi-C sequencing in understanding chromosome architecture
Conventional genomics research has historically concentrated on the linear arrangement of gene sequences. However, the genome's structural organization within cells is not a simple linear arrangement; rather, it is a complex three-dimensional structure in which genes do not exist in isolation but rather interact with each other. In this three-dimensional space, genes are intertwined and interact with each other. Hi-C technology facilitates the construction of a three-dimensional "map" of the genome by capturing the physical contact information between different regions of the genome, thereby elucidating the intricacies of genome folding.
Concomitantly, genes do not merely exist in isolation; rather, they are subject to regulation by numerous factors. Hi-C technology facilitates a more profound comprehension of the intricate interplay between genes and genes, as well as between genes and regulatory elements. These interactions are of paramount importance for cellular functions, developmental processes, and the onset of disease. A notable example is the frequent association of diseases such as cancer and diabetes with abnormalities in gene regulation. Hi-C technology offers researchers more intuitive tools to explore the mechanisms by which these abnormalities occur.
Service you may intersted in
Learn More:
Since its introduction in 2009, Hi-C technology has become a core tool for analyzing 3D genome structure. By capturing the spatial interaction information of chromosomes in the nucleus, Hi-C not only reveals the folding law of the genome but also provides a new perspective for understanding gene regulation, disease mechanism, and evolution. Currently, its application is mainly focused on the exploration of 3D genome structure and gene regulatory mechanisms.
3D genome structure and chromosome conformation
Although traditional genomics has historically focused on linear DNA sequences, Hi-C technology has emerged as a novel approach that visualizes chromosome interactions on a genome-wide scale. This technology combines high-throughput sequencing with cross-linking technology, providing a comprehensive view of genome interactions. Recent findings have revealed that the genome is not randomly distributed within the nucleus, but rather forms a multilayered folded structure. For instance, the genome is divided into active A compartments and silent B compartments, corresponding to open and dense chromatin states, respectively. Chromosomes are further partitioned into topologically associated domains (TADs) of approximately 0.1-1 Mb in size, and genes within these structural domains tend to be co-regulated. Furthermore, the presence of loops in the chromatin structure, facilitated by CTCF proteins and cohesins, enables the interaction between distal regulatory elements (e.g., enhancers) and promoters, thereby modulating gene expression. A study published in Cell in 2014 employed Hi-C technology to elucidate the dynamics of TADs during developmental processes, providing substantial evidence for comprehending cell fate decisions. These observations imply that the three-dimensional genomic architecture plays a pivotal role in the execution of gene function.
Genomic interactions and their role in gene regulation
Hi-C technology has further revealed the tight correlation between the three-dimensional structure and function of the genome. Many genetic mutations in non-coding regions, although not directly affecting gene sequences, may disrupt chromatin spatial interactions and lead to disease. For example, multiple mutations associated with TAD boundary disruption have been identified in schizophrenia and cancer. Integrating Hi-C with epigenetic data, such as ChIP-seq, has revealed a high correlation between histone modifications, including H3K4me3, and DNA methylation with respect to chromatin spatial conformation. Furthermore, Hi-C mapping of different cell types has revealed significant differences in genome folding patterns among specialized cells, such as immune cells and neurons, which are closely related to their functional specificity. These studies contribute to a more profound comprehension of gene regulatory mechanisms and offer novel molecular markers for disease diagnosis.
Recent advancements in single-cell technology and multi-omics integration have led to the expansion of Hi-C applications, signifying a transition from fundamental research to clinical and personalized medicine.
Single-cell Hi-C in personalized medicine
Traditional Hi-C relies on population cell data, masking cellular heterogeneity. The advent of single-cell Hi-C technologies (e.g., scHi-C reported in Nature in 2021) has enabled the resolution of genomic conformation at the individual cell level. In the context of cancer, where the three-dimensional structure of the genome may exhibit significant variations among cells within a tumor, single-cell Hi-C can facilitate the identification of drug-resistant clones or subpopulations of cells with high metastatic potential. For instance, by examining the chromatin conformation of a specific cell type, the potential for drug targets or epigenetic treatment responses can be ascertained. This approach, for instance, can be utilized for the screening of epigenetic drug-sensitive populations in leukemia. Such individualized analysis provides a novel technical foundation for precision medicine.
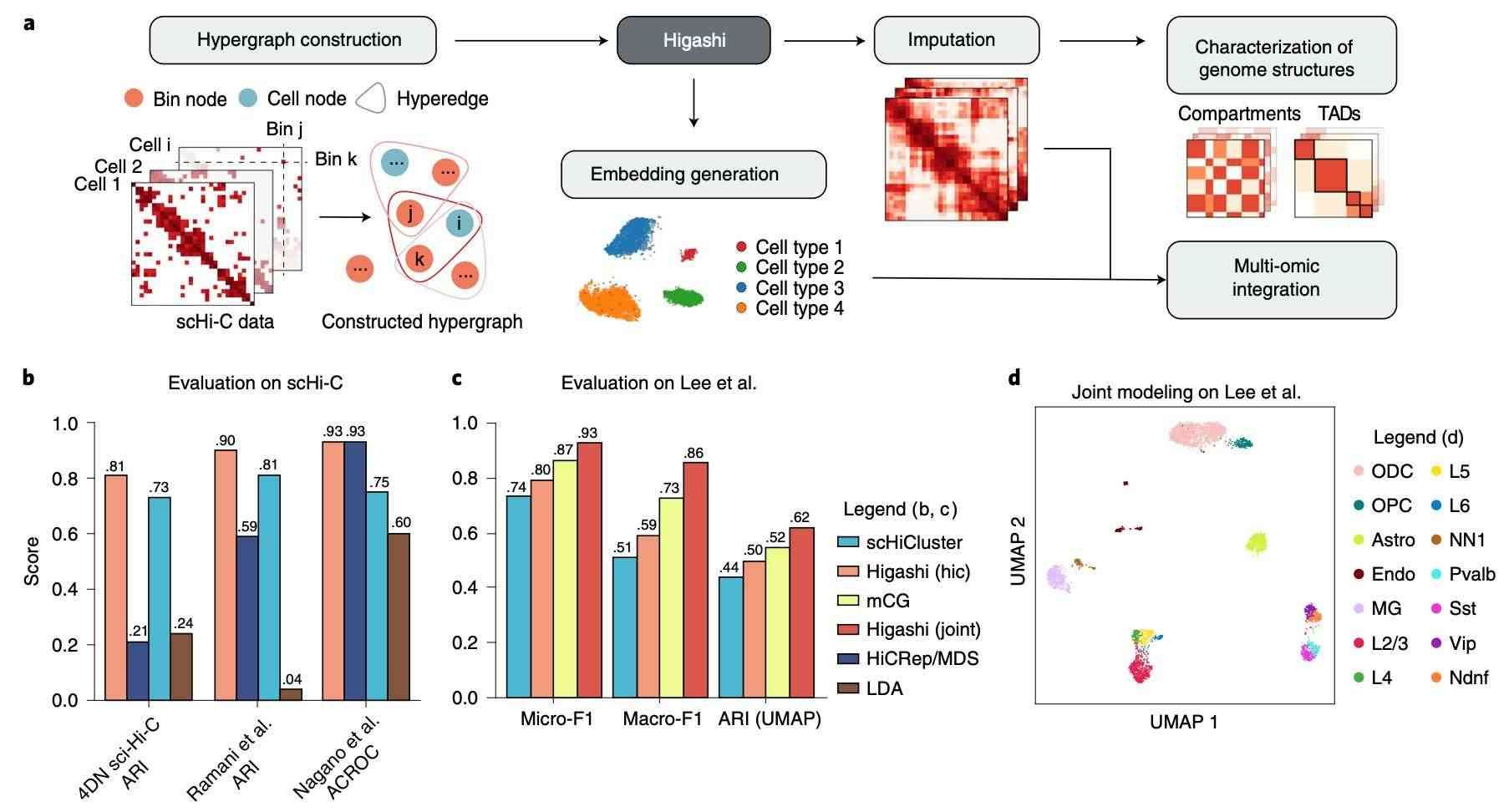 Overview of the Higashi framework for scHi-C analysis (Zhang et al., 2022)
Overview of the Higashi framework for scHi-C analysis (Zhang et al., 2022)
Multi-omics integration with Hi-C data (e.g., transcriptomics, epigenomics)
The integration of Hi-C data with the transcriptome, epigenome, and proteome (Multi-Omics) has emerged as a prominent area of research interest. Through the combination of ATAC-seq (open chromatin) and RNA-seq (gene expression), researchers have developed a three-dimensional, genome-driven regulatory network. In a study published in Science, researchers identified hundreds of non-coding regulatory elements associated with cancer metastasis by integrating Hi-C with CRISPR screening. Furthermore, in the context of cellular differentiation or stress responses, the integration of time-series multi-omics data can unveil causal relationships between chromatin remodeling and functional changes, thereby providing a more comprehensive framework for elucidating complex life processes.
Hi-C in developmental biology: implications for stem cell research and embryogenesis
Hi-C technology provides a level of spatial and temporal resolution of developmental processes that has not been previously achieved. In the context of mouse and human embryo studies, Hi-C reveals a global remodeling of chromosomes during syncytial genome activation (ZGA), as well as the spatial segregation of paternal and maternal chromosomes. In the process of generating induced pluripotent stem cells (iPSCs), the reclassification of TADs is closely related to the efficiency of cellular reprogramming. The targeted modulation of these structures has the potential to optimize strategies in the field of regenerative medicine. Furthermore, the integration of organoid culture with Hi-C technology has enabled the recreation of three-dimensional genomic dynamics in organ development, providing novel models for the study of congenital malformations. These advancements underscore the fundamental significance of Hi-C in the realm of developmental biology.
Service you may intersted in
Despite the substantial advancements in Hi-C technology, challenges persist regarding its resolution, throughput, and dynamic analysis capability. Future development will prioritize technological innovation and interdisciplinary integration.
Long read-length sequencing technologies (e.g., PacBio and Oxford Nanopore) have the potential to enhance Hi-C assembly and annotation across repetitive sequence regions, particularly in complex regions such as filaments and telomeres. In addition, CRISPR-Cas9-mediated genome editing technologies can target the disruption of specific chromatin loops or TAD boundaries to validate the biological functions of three-dimensional genomic structures. Furthermore, the integration of super-resolution microimaging technologies, such as light-sheet microscopy, with Hi-C is anticipated to facilitate single-molecule-level verification of spatial interactions. The concurrent application of these technologies is expected to significantly enhance the precision and depth of 3D genome research.
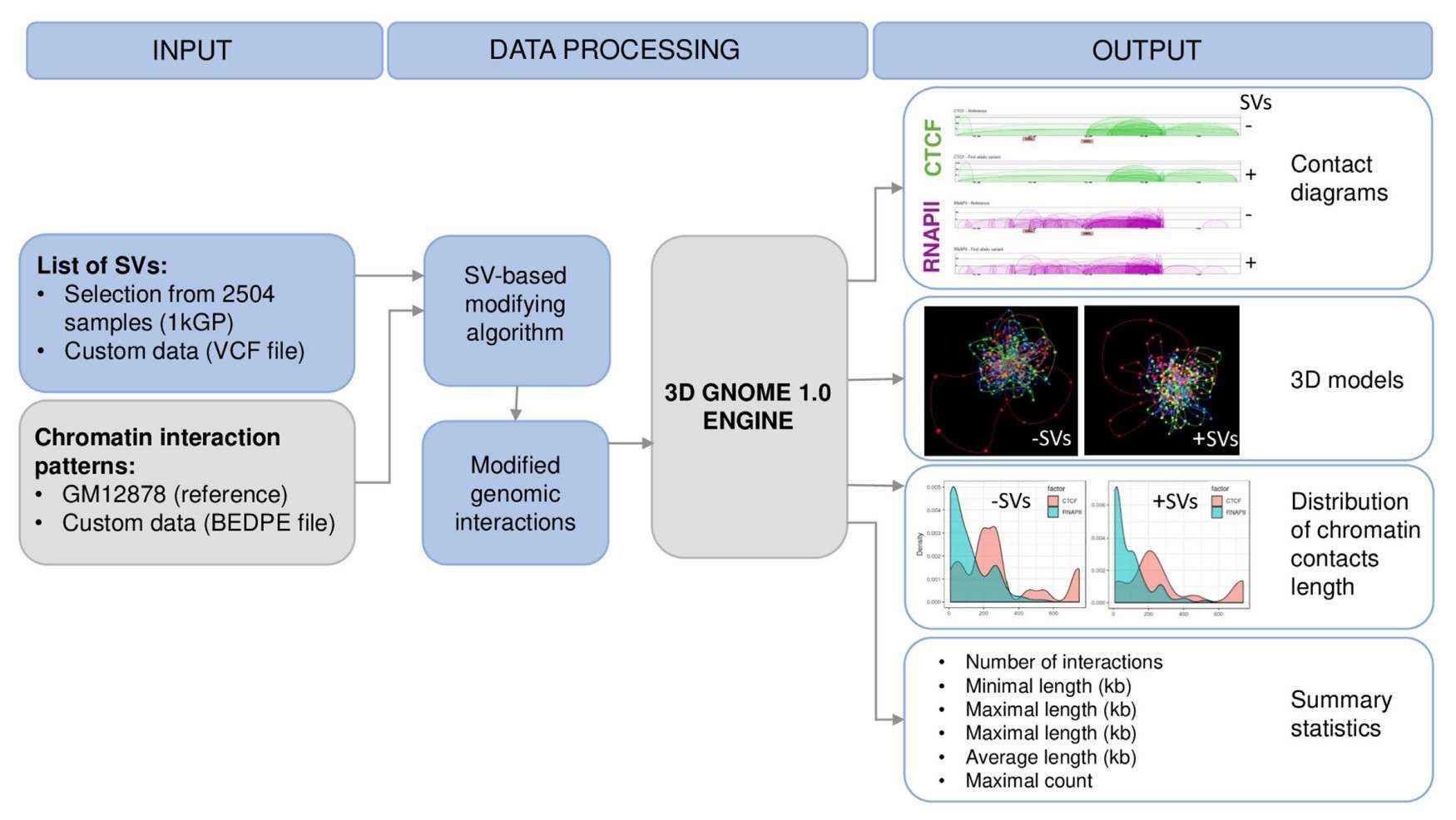 A schematic representation of the workflow of 3D-GNOME 2.0 (Wlasnowolski et al., 2020)
A schematic representation of the workflow of 3D-GNOME 2.0 (Wlasnowolski et al., 2020)
The advent of novel technologies, such as nanopore Hi-C, is anticipated to enhance resolution to the kilobase level while concurrently reducing expenses and fostering large-scale population studies. The evolution of live cell Hi-C technology holds the potential to facilitate real-time observation of chromatin dynamics during the cell cycle, DNA damage repair, and other processes. In terms of clinical translation, the establishment of 3D genomic databases (e.g., 3DGNOME 2.0) and the combination of artificial intelligence can predict the risk of diseases or drug targets, and ultimately realize "3D genomic medicine." These technological breakthroughs will push genomics from descriptive research to functional and applied exploration.
Hi-C technology has redefined genomics from a three-dimensional perspective, and its applications have extended from basic research to disease diagnosis, drug development, and personalized medicine. By resolving the spatial organization of chromosomes, Hi-C reveals the complex network of gene regulation and provides new ideas for the study of the mechanism of cancer, developmental abnormalities, and other diseases. The advent of single-cell technology, multi-omics integration, and innovative sequencing platforms has catalyzed the evolution of 3D genomics, promising to profoundly elucidate the intricacies of life processes and furnish novel instruments for precision medicine. Notwithstanding the prevailing technical obstacles, Hi-C is poised to usher genomics into a novel era of "three-dimensionalization," thereby realizing the aspiration of transitioning from "sequence" to "structure" to "function" and "functionality" of the genome.
References
Terms & Conditions Privacy Policy Copyright © CD Genomics. All rights reserved.