As a golden standard technique, Sanger sequencing exercises impeccable precision in sequence determination down to single base-pair resolution. This characteristic renders it exceptionally suitable for targeted sequencing and confirmatory analyses. CD Genomics provides top-tier Sanger sequencing services, delivering comprehensive sequence analysis for a variety of samples, including plasmid DNA, PCR products, DNA fragments, large DNA molecules and etc. Leveraging the latest advancements in Sanger sequencing technology, we ensure the accuracy and reliability of sequencing outcomes.
The Introduction of Sanger Sequencing
Sanger sequencing, also known as the "chain termination method", is a method of DNA sequencing based on the selective incorporation of chain-terminating dideoxynucleotides by DNA polymerase during in vitro DNA replication. Developed by two time Nobel Laureate Frederick Sanger and his colleagues in 1977, hence the name the Sanger Sequence, it was the most widely used sequencing method for approximately 40 years.
More recently, Sanger sequencing has been replaced by Next-Generation sequencing methods, especially for large-scale, automated genome analyses. However, Sanger remains useful for sequencing single genes or amplicon targets of up to 100 base pairs in length, for projects involving 96 or fewer samples, for microbial identification and gene fragment analysis, and for analyzing short tandem repeats. Moreover, Sanger is considered the "gold standard" sequencing method for validating the sequence of specific genes.
Sanger sequencing also is the most widely used testing platform for mutation detection in various cancer settings, as it provides a comprehensive examination of all genetic aberrations in the sample material. Sanger sequencing has proven useful for assessing the presence or absence of recurrent single nucleotide mutations or small insertions/deletions in oncogenes and tumor suppressor genes in surgically resected pathology specimens.
If you want to learn more about Sanger sequencing, you can refer to our articles "Sanger Sequencing: Introduction, Workflow, and Applications" and "Sanger Sequencing: Introduction, Principle, and Protocol."
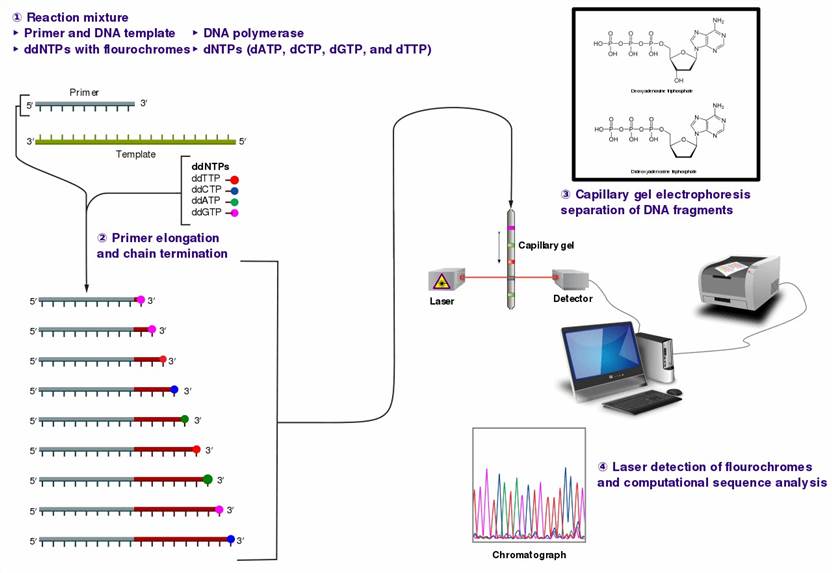 Figure 1. The Sanger (chain-termination) method for DNA sequencing.
Figure 1. The Sanger (chain-termination) method for DNA sequencing.
What is the Principle of Sanger Sequencing
The crux of Sanger sequencing rests upon the functional capacity of DNA polymerase, which is commanded to generate nascent DNA strands. This procedure, however, involves the strategic assimilation of distinct dideoxynucleotides (ddNTPs) within the reaction course. A noteworthy feature of these ddNTPs is their absence of a 3' hydroxyl faction, a factor that initiates a cessation in the DNA synthesis. A systematic evaluation of the dimensions and arrangement of the DNA fragments permits the deduction of the nucleotide sequencing inherent to the DNA under investigation.
In the realm of Sanger sequencing, despite nuanced disparities between sequencing procedures involving plasmid DNA and PCR products, the fundamental principles remain akin.
Plasmid DNA Sanger Sequencing:
In plasmid DNA Sanger sequencing, the process commences with the extraction of the target plasmid DNA, followed by the design of primers to delineate the regions slated for sequencing. Subsequently, a DNA polymerase-initiated extension reaction transpires, whereby a nascent DNA chain is synthesized, commencing from the primers. Diverging from conventional PCR, the infusion of specialized dideoxynucleotides (ddNTPs) during the reaction introduces sporadic substitutions of conventional deoxynucleotides (dNTPs), culminating in the termination of DNA chain elongation. Sequentially, electrophoretic analysis of the reaction products is conducted, enabling the determination of the size order of DNA fragments through the interpretation of electropherograms, thus furnishing the foundational nucleotide sequence of the plasmid DNA.
Regarding plasmid DNA sequencing services, CD Genomics also offers Complete Plasmid DNA Sequencing. Complete Plasmid DNA Sequencing has its unique advantages compared to Sanger sequencing.
PCR Product Sanger Sequencing:
For Sanger sequencing of PCR products, the process initiates with the amplification of the target DNA segments via PCR. Following amplification, purification, and concentration steps, primer design ensues, preceding DNA polymerase-mediated extension reactions. Analogous to plasmid DNA sequencing, the incorporation of ddNTPs is pivotal in curtailing DNA strand elongation. Subsequently, electrophoretic analysis is conducted, elucidating the sequence of nucleotide bases within the PCR products through the interpretation of electropherograms.
What are the Advantages of Sanger Sequencing
- High Quality - DNA sequencing read lengths up to ~800 bases
- Highly-Trained Technical Support-Our team provides accurate, reliable data
- Rapid Turnaround Time
- Low-Cost
- Free advice and trouble shooting
What are the Applications of Sanger Sequencing
- HSP (hairpin, stemloop, palindrome) for problematic templates
- Fragment analysis applications
- Microsatellites
- AFLP (amplified fragment length polymorphism)
- Single nucleotide polymorphism and mutation detection
- Chromosomal insertion and deletion (INDEL)
- 16S Microbial Identification
- Defining the mutational spectrum of a tumor
- Identifying a constitutional variant in diagnostic testing
Sanger Sequencing Workflow
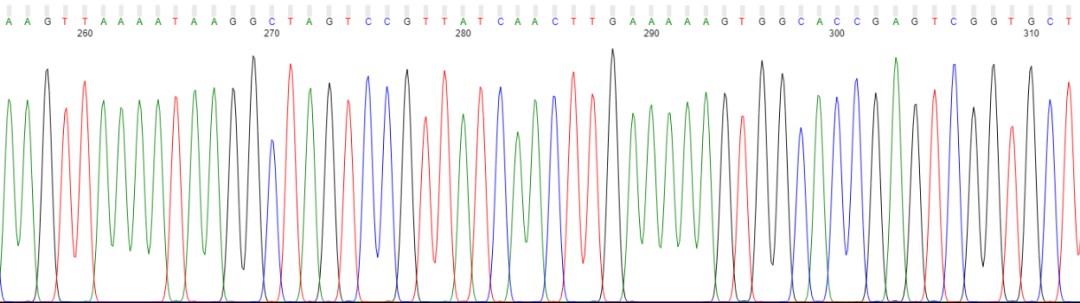
As a benchmark technology, Sanger sequencing delivers precise sequence determination at single-base resolution, making it superbly suited for targeted sequencing and confirmation analysis. First and foremost, DNA is extracted from the sample and impurities are eliminated. Two primers are then designed to direct the synthesis of DNA by the polymerase. A DNA polymerisation reaction ensues where tailored ddNTPs are introduced to conclude DNA synthesis. Following this, reaction products are differentiated through gel electrophoresis, and the resulting electropherogram is analysed based on size sequence. The culmination of this process is the definitive establishment of the nucleotide sequence of the target DNA.
CD Genomics provides Plasmid DNA Sanger Sequencing and PCR Product Sanger Sequencing.
Service Specification
Sample Requirements
|
|
Sequencing Strategy
|
|
Key Data
|
Analysis Pipeline
Deliverables
The results are returned in two forms:
- Raw sequence file (.seq file)
- Raw trace (.ab1 file)
More than a decade of experience in Sanger sequencing makes CD Genomics the most trusted company for your DNA sequencing projects. Get high standard results with short delivery times and personalized customer support. Our highly-trained technical support provide you the best service on the market. If you have additional requirements or questions, please feel free to contact us.
1. What is Sanger sequencing?
Sanger sequencing, colloquially classed as a pioneering technique within the realm of DNA sequencing, traces its roots back to its eponym, Frederick Sanger, and his associates who first conceived this method in 1977. This strategy takes pride of place amongst the earliest methodologies introduced in the expansive field of genomics, chiefly utilized for deciphering the precise sequencing of nucleotide bases integral to the structure of a DNA sequence.
2. What samples are suitable for Sanger sequencing?
Sanger sequencing exhibits a broad application spectrum, catering to diverse DNA samples, such as genomic DNA, plasmid DNA, and PCR products. Given its extensive utility, it finds application in numerous domains, ranging from fundamental biological research, genetic explorations and clinical diagnostics, among others.
3. What are the limitations of Sanger sequencing?
While Sanger sequencing is renowned for its precision and high fidelity, it falls short on several fronts when juxtaposed with next-generation sequencing methods. Characterized by its lower throughput, more protracted turnaround times, and inflated costs, Sanger sequencing is less advantageous for undertaking large-scale genomic sequencing and high-throughput sequencing ventures.
4. How is Sanger sequencing applied in genomics research?
Sanger sequencing finds myriad applications in genomic inquiries, encompassing gene mutation studies, identification and characterization of single nucleotide polymorphisms (SNPs), gene expression profiling, and studies honing in on evolutionary pathways. It bestows research scientists with a pivotal tool to harness genomic data, thereby answering intricate biological, medical, and environmental queries.
5. How does Sanger sequencing differ from other sequencing technologies?
When set side-by-side with next-generation sequencing technologies, Sanger sequencing stands out for its higher resolution and extended read lengths, albeit its throughput is comparatively modest and its cost profile steeper. Emerging sequencing technologies, widely categorized as NGS methodologies, typically manifest heightened throughput and expedited processing times, making them ideal for large-scale genome sequencing and investigations at the population level.
Comparing Whole-Genome Sequencing with Sanger Sequencing for spa Typing of Methicillin-Resistant Staphylococcus aureus
Journal: Journal of clinical microbiology
Impact factor: 11.677
Published: December 2014
Background
The article highlights the enduring prevalence of MRSA in hospitals and its increasing incidence in communities worldwide. It discusses the widespread adoption of spa typing for MRSA analysis, facilitated by the Ridom StaphType software. Additionally, it outlines the implementation of whole-genome sequencing (WGS) at Hvidovre Hospital, Denmark, since 2013 to enhance MRSA outbreak investigations and surveillance. The study aims to assess the reliability of MRSA spa typing by comparing WGS with Sanger sequencing results.
Methods
- One MRSA isolate per patient
- Multiplex real-time PCR assay
- Whole genome sequencing
- Sanger sequencing
- Genome assembly
- Sequence comparison
Results
The investigation scrutinized 699 Methicillin-resistant Staphylococcus aureus (MRSA) isolates, revealing a remarkable concordance of 97% in spa types between Sanger sequencing and WGS. Within this cohort, 136 distinct spa types were discerned, with predominant types including t002, t008, t019, and t304. Discrepancies in spa types between the two methodologies were observed in 19 isolates, primarily attributable to variations in the number of 24-bp repeats. Notably, one case of discordance stemmed from suboptimal assembly quality and isolate contamination, necessitating resequencing and the identification of multiple spa types within a single sample. Instances of inadequate sequencing outcomes or assembly challenges during initial WGS analyses prompted subsequent resequencing efforts, ultimately yielding successful spa typing for all isolates. These findings underscore the complementary roles of Sanger sequencing and WGS in MRSA spa typing, with the latter offering advantages in efficiency and resolution, albeit accompanied by certain technical intricacies demanding resolution.
Table 1 Isolates with different spa types by WGS and Sanger sequencing
| spa type/ST by WGS | spa type by Sanger sequencing | spa repeats by WGS | spa repeats by Sanger sequencing |
| t015/ST45 | t026 | 08-16-02-16-34-13-17-34-16-34 | 08-16-34 |
| t032/ST22 | t1249 | 26-23-23-13-23-31-29-17-31-29-17-25-17-25-16-28 | 26-23-23-13-23-23-13-23-31-29-17-31-29-17-25-17-25-16-28 |
| t086/ST88 | t690 | 07-12-21-17-13-13-13-34-34-34-33-34 | 07-12-21-17-13-13-34-34-34-33-34 |
| t186/ST88 | t690 | 07-12-21-17-13-13-34-34-33-34 | 07-12-21-17-13-13-34-34-34-33-34 |
| t304/ST6 | t197 | 11-10-21-17-34-24-34-22-25 | 11-10-34-24-34-22-25 |
| t355/ST152 | t595 | 07-56-12-17-16-16-33-31-57-12 | 07-56-12-17-16-16-33-31-57-31-57-12 |
| t359/ST97 | t267 | 07-23-12-21-17-34-34-33-34 | 07-23-12-21-17-34-34-34-33-34 |
| t630/NA | t304 | 08-16-02-16-34-17-34-16-34 | 11-10-21-17-34-24-34-22-25 |
| t670/ST22 | t5177 | 26-23-23-13-23-29-17-25-17-25-16-28 | 26-23-23-13-23-29-17-31-29-17-31-29-17-25-17-25-16-28 |
| t718/ST22 | t1249 | 26-23-23-23-13-23-31-29-17-31-29-17-25-17-25-16-28 | 26-23-23-13-23-23-13-23-31-29-17-31-29-17-25-17-25-16-28 |
| t728/ST45b | t015 | 08-16-34-16-34 | 08-16-02-16-34-13-17-34-16-34 |
| t790/ST22 | t022 | 26-23-13-23-31-29-17-25-17-25-16-28 | 26-23-13-23-31-29-17-31-29-17-25-17-25-16-28 |
| t934/ST80 | t1198 | 07-23-12-34-34-34-34-33-34 | 07-23-12-34-34-34-34-34-33-34 |
| t1028/ST78 | t237 | 07-34-33-34 | 07-34-34-33-34 |
| t4699/ST93 | t3949 | 11-17-16-16-25 | 11-17-23-17-17-17-16-16-25 |
| t5090/ST130 | t843 | 04-82-17-16-17 | 04-82-17-25-17-25-25-16-17 |
| t5608/ST5 | t002 | 26-23-17-34-17-13-17-20-17-12-17-16 | 26-23-17-34-17-20-17-12-17-16 |
| t3119/ST8 | t1774 | 11-19-19-19-12-05-17-34-24-34-22-25 | 11-19-19-12-05-17-34-24-34-22-25 |
Conclusion
Results indicated a 97% agreement between spa types obtained by WGS and conventional methods. Although nineteen isolates showed discordant spa types, primarily due to differences in the number of 24-bp repeats, these discrepancies were deemed inconsequential for outbreak investigations. This is because all epidemiologically linked isolates, irrespective of spa type, are included in single nucleotide polymorphism (SNP) analysis, revealing their close relatedness. Ultimately, the study concludes that WGS is a reliable method for determining MRSA spa types.
Reference:
- Bartels M D, Petersen A, Worning P, et al. Comparing whole-genome sequencing with Sanger sequencing for spa typing of methicillin-resistant Staphylococcus aureus. Journal of clinical microbiology, 2014, 52(12): 4305-4308.
Here are some publications that have been successfully published using our services or other related services:
The HLA class I immunopeptidomes of AAV capsid proteins
Journal: Frontiers in Immunology
Year: 2023
Isolation and characterization of new human carrier peptides from two important vaccine immunogens
Journal: Vaccine
Year: 2020
Change in Weight, BMI, and Body Composition in a Population-Based Intervention Versus Genetic-Based Intervention: The NOW Trial
Journal: Obesity
Year: 2020
Sarecycline inhibits protein translation in Cutibacterium acnes 70S ribosome using a two-site mechanism
Journal: Nucleic Acids Research
Year: 2023
Identification of a Gut Commensal That Compromises the Blood Pressure-Lowering Effect of Ester Angiotensin-Converting Enzyme Inhibitors
Journal: Hypertension
Year: 2022
A Splice Variant in SLC16A8 Gene Leads to Lactate Transport Deficit in Human iPS Cell-Derived Retinal Pigment Epithelial Cells
Journal: Cells
Year: 2021
See more articles published by our clients.


 Sample Submission Guidelines
Sample Submission Guidelines
