We use cookies to understand how you use our site and to improve the overall user experience. This includes personalizing content and advertising. Read our Privacy Policy
Hi-C is a high-throughput genomic and epigenomic technique designed to capture the three-dimensional (3D) conformation of chromatin within the nucleus. Chromatin, which packages long DNA strands in a confined nuclear volume, plays a crucial role in biological functions at both the gene and global nuclear levels. Understanding chromatin organization is essential for insights into gene regulation, chromosome morphogenesis, genome stability, and transmission, as well as the biophysics of chromatin and pathologies related to genome instability or nuclear morphology.
The Hi-C protocol initiates with the crosslinking of chromatin within intact cells using formaldehyde. This crucial step stabilizes the spatial interactions between DNA and its associated proteins, effectively "freezing" the three-dimensional architecture of the genome at a specific point in time. Subsequent to crosslinking, the chromatin is digested with a restriction enzyme that cleaves the DNA at precise recognition sites. This enzymatic digestion produces a diverse library of restriction fragments, laying the groundwork for further detailed analysis.
After enzymatic digestion, the resultant DNA fragments undergo ligation under diluted conditions to facilitate the joining of fragments that were closely positioned within the nucleus. This critical step results in the formation of chimeric DNA molecules, wherein the junctions signify direct interactions between distal genomic loci. Subsequently, the ligated DNA is purified to eliminate proteins and other cellular constituents.
Following purification, the ligation products undergo sequencing using high-throughput technologies. Subsequently, the sequencing reads are aligned against the reference genome to pinpoint the locations of the interacting fragments. Quantification of these interactions allows for the creation of a contact matrix, which illustrates the spatial proximity between distinct genomic regions.
The resolution of Hi-C data hinges upon the complexity of the ligation product library, which in turn is influenced by the number of cells utilized in the experiment. Achieving a high-complexity library typically necessitates millions of cells to comprehensively capture interactions spanning the entire genome. This requirement is especially critical for investigating the overarching spatial organization of expansive mammalian genomes.
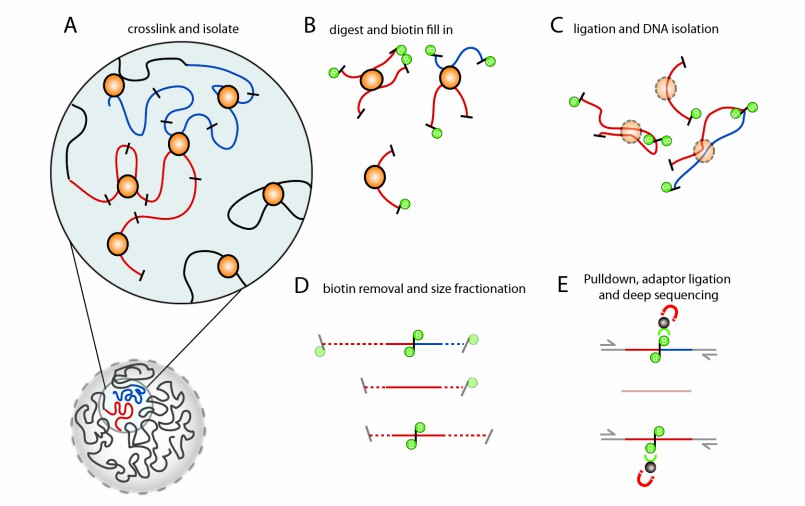 Figure 1. Overview of Hi-C technology (Belton et al. 2013)
Figure 1. Overview of Hi-C technology (Belton et al. 2013)
Service you may intersted in
The advantages of Hi-C technology are multifaceted and transformative:
Hi-C technology provides comprehensive genome-wide interaction maps, offering a holistic view of chromatin organization. By capturing interactions between genomic loci, Hi-C reveals the intricate spatial relationships that define the 3D architecture of the genome.
Modern Hi-C protocols, coupled with advanced bioinformatics tools, achieve high resolution and sensitivity. This capability enables the detection of both short-range interactions and subtle chromatin features, enhancing precision in mapping genomic interactions across various scales.
Hi-C enables the detailed characterization of 3D genomic structures, including Topologically Associating Domains (TADs), chromatin loops, and regulatory interactions critical for gene expression. This methodological approach significantly contributes to understanding how spatial genome organization influences genome function and stability.
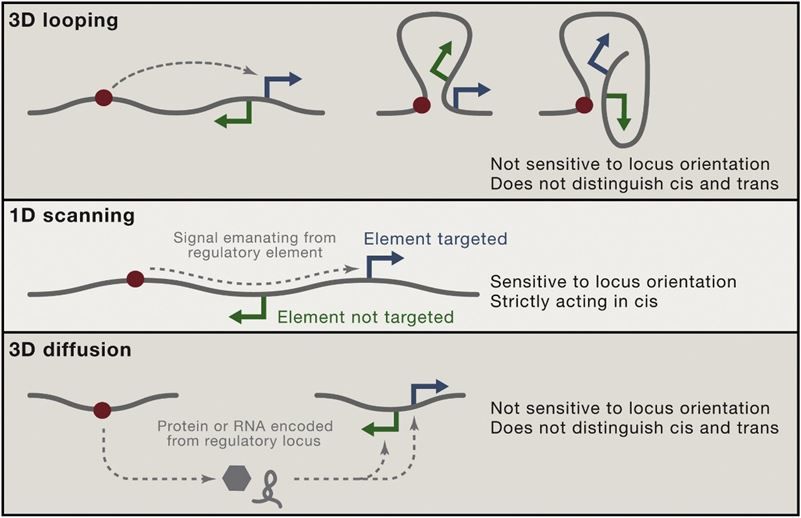 Figure 2. Chromosomal Communication (Job, 2016)
Figure 2. Chromosomal Communication (Job, 2016)
Hi-C studies reveal that the spatial proximity of genomic elements correlates with functional interactions, such as enhancer-promoter looping, offering mechanistic insights into gene regulation mechanisms. This understanding elucidates how specific genomic configurations facilitate or hinder regulatory interactions crucial for cellular processes and developmental programs.
Hi-C has emerged as a pivotal tool in studying chromatin alterations associated with diseases. By uncovering aberrant chromatin structures and patterns of genome instability, Hi-C contributes to identifying potential disease mechanisms and therapeutic targets. Its applications in disease research span from elucidating genetic predispositions to exploring epigenetic modifications and their implications in disease progression.
Hi-C and ChIP-seq are both advanced high-throughput sequencing techniques, each offering unique yet complementary insights into the genome's organization and regulation. Grasping the distinctions and applications of these methodologies can significantly advance genomic research.
Data Output:
Hi-C: Produces interaction maps that illustrate the spatial proximity of chromatin regions.
ChIP-seq: Generates peak maps showing specific binding sites of proteins on the DNA.
Resolution:
Hi-C: Provides a broad overview of genome organization, with resolution varying based on sequencing depth and protocol.
ChIP-seq: Offers high resolution at specific protein binding sites, providing precise localization of protein-DNA interactions.
Complementary Insights:
Hi-C and ChIP-seq Integration: By combining Hi-C with ChIP-seq data, researchers can investigate the relationship between chromatin structure and protein binding. For example, integrating these datasets can reveal how transcription factor binding within TADs influences gene expression and chromatin organization.
Complex Gene Regulation: The integration helps to dissect complex regulatory mechanisms, such as those involving Runx1 in hematopoietic development, by correlating chromatin interactions with transcription factor binding and gene expression.
Hi-C and ChIP-seq are both essential tools in the genomics toolkit, each providing unique insights into the genome's structure and function. Hi-C excels in mapping the three-dimensional organization of chromatin, while ChIP-seq is indispensable for identifying specific protein-DNA interactions. Together, these techniques enable a comprehensive understanding of gene regulation, chromatin dynamics, and the mechanisms underlying various biological processes and diseases.
Hi-C can uncover the chromatin interactions. The study (Lieberman-Aiden et al. 2009) mapped chromatin contacts in human cells, unveiling the spatial organization of chromosomes into TADs and revealing long-range interactions crucial for gene regulation.
Hi-C plays a vital role in elucidating chromatin folding and compartmentalization. Gibcus and Dekker (2013) underscored the partitioning of chromatin into A and B compartments, where A compartments are linked to active euchromatin and B compartments to repressive heterochromatin.
Hi-C can discern chromatin loops and enhancer-promoter interactions. Dixon et al. (2012) employed this function. Their investigation in mouse embryonic stem cells highlighted the regulatory significance of chromatin looping in governing gene expression and cellular differentiation.
Hi-C have been explored the application in understanding disease mechanisms. Recent studies by Javierre et al. (2020) and Lupiáñez et al. (2015) demonstrated how aberrant chromatin interactions identified through Hi-C can contribute to disease pathogenesis, offering insights into potential therapeutic targets.
Recent advancements in Hi-C protocols have notably enhanced resolution and sensitivity in chromatin interaction studies. As detailed by Ramani et al. (2016), their research emphasizes the application of in situ Hi-C, which achieves unprecedented resolution in capturing chromatin interactions. This methodological refinement enables the precise detection of short-range interactions and subtle chromatin features that were previously challenging to resolve.
Hi-C data can correlate spatial proximity with gene regulation. A study by Rao et al. (2014) provided functional insights into this function. Their analysis revealed that spatial clustering of genes and regulatory elements influences gene expression patterns, shedding light on the functional implications of chromatin organization detected by Hi-C.
These scientific examples exemplify the varied applications and transformative impact of Hi-C technology in decoding the 3D genome landscape. Through rigorous experimentation and meticulous data analysis, Hi-C continues to elucidate the complexities of chromatin structure and function, thereby laying the groundwork for pioneering discoveries in genomics.
Despite its powerful capabilities, analyzing Hi-C data is complex and traditionally required a skilled computational biologist. The process involves numerous steps, including processing ligation-joined reads, performing alignment, pairing reads, removing duplicates, and performing normalizations. Additionally, high-resolution chromatin contact maps for the human genome require billions of Hi-C sequencing reads, making the analysis even more challenging.
To address these challenges, two companion tools, Juicer and Juicebox, which significantly ease the computational burden of Hi-C data analysis. Juicer automates the entire processing pipeline, from raw reads to usable contact coordinates, incorporating parallel processing to reduce the time required. It also includes multiple normalization techniques, allowing for the comparison of different methods and providing flexibility for downstream analysis.
Hi-C technology has revolutionized our understanding of genome organization and function. By capturing the spatial interactions between genomic regions, Hi-C provides a comprehensive view of the 3D architecture of the genome. This technique has wide-ranging applications, from studying chromatin structure and gene regulation to uncovering disease mechanisms and evolutionary processes. As Hi-C technology continues to advance, it will undoubtedly provide deeper insights into the complex interplay between genome structure and function, driving further discoveries in genomics and biomedical research.
References
Terms & Conditions Privacy Policy Copyright © CD Genomics. All rights reserved.