What is Liquid Biopsy?
Liquid biopsy analyzes body fluids and diagnoses diseases by sampling patients' blood, cerebrospinal fluid, saliva, pleural fluid, ascites, urine, etc. It can avoid the influence of tissue heterogeneity on molecular typing of tumors to a certain extent and can be used for early cancer screening and postoperative recurrence monitoring. At present, blood-based liquid biopsy is the main research direction, mainly detecting free circulating tumor DNA (ctDNA), circulating tumor cells (CTCs) and exosomes in blood.
The ctDNA with tumor tissue mutation information is released from tumor cells into the blood, and liquid biopsy can detect various types of mutations including point mutations (SNV), short fragment insertion deletions (Indel), fusion genes (Fusion), copy number variants (CNV), etc.
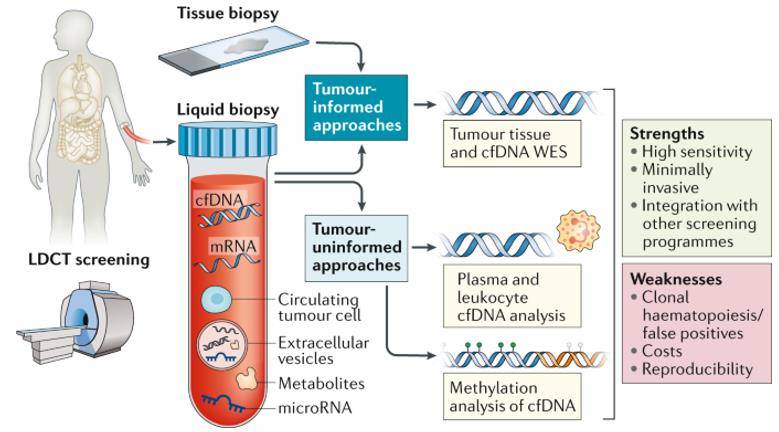 Liquid biopsy for early stage lung cancer. (Rolfo et al., 2020)
Liquid biopsy for early stage lung cancer. (Rolfo et al., 2020)
The Advantages of Liquid Biopsy
Compared with tissue biopsy methods, liquid biopsy uses innovative and minimally invasive technology, which makes both the diagnostic and therapeutic stages of biopsy simpler, faster, and easier to repeat and monitor dynamically, and is not affected by tumor heterogeneity and can reflect the overall tumor variant status to a certain extent. It also provides an effective way to understand the tumor genetic variant status for patients who have difficulty in obtaining biopsy tissues or whose tissue samples are not sufficient for genetic testing. All this information can help provide reference basis for doctors and help patients to develop personalized treatment plans, which can ultimately benefit them the most in precision medicine.
Safe and Minimally Invasive
Liquid biopsies are minimally invasive and can reduce the side effects and recovery time of the invasive operation, and are less painful, highly operable and reproducible, which can help improve the compliance of the patient and enable dynamic monitoring of recurrence, efficacy or drug resistance.
Earlier Detection of Tumor Tissue
Tissue biopsy mainly relies on imaging, but in the early stage of tumor, it is difficult to detect clear tumor lesions by imaging. Liquid biopsy can detect tumors earlier than imaging by capturing DNA fragments released from tumor cells into the bloodstream, thus enabling early screening of tumors and postoperative recurrence risk assessment.
Spatial and Temporal Heterogeneity and Time-Sensitive
Tissue biopsy can only give a "snapshot" of specific parts of the tumor at specific times, which has certain limitations, but ctDNA is derived from tumor tissues of all sites, which can obtain more tumor-related gene mutation data during tumor progression and can reflect the whole tumor picture more comprehensively and accurately. Meanwhile, liquid biopsy markers have a short half-life and are time-sensitive, which can be used as a dynamic tool to track treatment response within a few hours and provide timely and effective information to the clinic.
What Are the Applications of Liquid Biopsy?
As one of the emerging technical tools for cancer diagnosis and treatment, research in the field of liquid biopsy is steadily growing and has made significant progress in the diagnosis and treatment of a variety of solid tumors. In 2016, the US FDA approved the first liquid biopsy technology for EGFR mutation detection in non-small cell lung cancer patients with EGFR exon 19 deletion or exon 21 L858R substitution mutation to guide the application of EGFR-targeted drugs. Since then, a large number of clinical studies have followed, and liquid biopsy technology has gradually moved from clinical research to practical application.
Drug Resistance Monitoring
The use of plasma ctDNA analysis can monitor several mechanisms of resistance to molecular targeted therapies. For example, ctDNA testing can indicate whether patients with non-small cell lung cancer are resistant due to the presence of EGFR exon 20 T790M mutation, which is an important guide for the selection of EGFR-targeted therapies. In addition to monitoring known resistance mechanisms, ctDNA testing can also be used to identify unknown resistance mechanisms and help select treatment options that target specific resistance mechanisms.
Prognostic Assessment
Microscopic residual disease (MRD), a current research hotspot in prognostic assessment, refers to molecular abnormalities of cancer origin that cannot be detected by conventional imaging or laboratory methods but can be detected by liquid biopsy after cancer treatment has achieved complete remission. Several solid tumor-related studies have shown that ctDNA-based MRD status can be used for prognostic assessment of patients after radical surgery and for recurrence risk prediction.
Early Tumor Screening
Compared with early-stage tumors, advanced-stage tumors have heavier tumor load and more ctDNA in the blood, so they are easier to be detected. The most important feature of early-stage tumors is that the tumor load is low, and the content of ctDNA in blood is also extremely low at this time, so it is difficult to meet the requirements of early diagnosis by ordinary tests for ctDNA mutation. At this time, testing the methylation level of plasma ctDNA is an effective aid for early tumor screening.
Reference:
-
Rolfo, Christian, and Alessandro Russo. "Liquid biopsy for early stage lung cancer moves ever closer." Nature Reviews Clinical Oncology 17.9 (2020): 523-524.
For research purposes only, not intended for clinical diagnosis, treatment, or individual health assessments.


 Sample Submission Guidelines
Sample Submission Guidelines
 Liquid biopsy for early stage lung cancer. (Rolfo et al., 2020)
Liquid biopsy for early stage lung cancer. (Rolfo et al., 2020)