CD Genomics has been providing the flexible and affordable whole exome sequencing service for couple of years. We employ Illumina HiSeq sequencing platform to obtain the genetic variations information in a more efficient way.
The Introduction of Whole Exome Sequencing
Human genome comprises approximately 3×109 bases, and contains approximately 180,000 coding regions (exome), constituting about 1.7% of a human genome. It is estimated that 85% of the disease-causing mutations occur in the exome. For this reason, sequencing of the whole exome has the potential to uncover higher yield of relevant variants at a far lower cost than whole genome sequencing. Whole exome sequencing is thought to be an efficient and powerful way to identify the genetic variants that affect heritable phenotypes, including important disease-causing mutations and natural variations that can be used to improve crops and livestock.
Whole Exome Sequencing utilizes exome capture technology to enrich exons, and then sequences these regions in a high-throughput manner. To be specific, DNA samples are first fragmented and biotinylated oligonucleotide probes (baits) are used to selectively hybridize to exome in the genome. Magnetic streptavidin beads are then used to bind to the biotinylated probes. The non-targeted portion of the genome is washed away, and the PCR is used to enrich the sample for DNA from the target region. Subsequently, the sample is sequenced by the Illumina HiSeq platform. This strategy can result in up to a 100-fold improvement in gene coverage for the human genome. The validated sequencing data are then used for variant analysis and clinical statements.
Advantages of Whole Exome Sequencing
- Lower cost and wide availability
- Increased sequence coverage (above 120X)
- Detection of coding single-nucleotide polymorphism (SNP) variants as sensitive as whole genome sequencing
- A smaller data set for faster and easier analysis compared to whole genome sequencing
- Medical and agricultural applications
Whole Exome Sequencing Workflow
CD Genomics employs the Illumina HiSeq system to provide the fast and accurate whole exome sequencing and bioinformatics analysis. Our highly experienced expert team executes quality management, following every procedure to ensure confident and unbiased results. The general workflow for whole exome sequencing is outlined below.

Service Specifications
Sample Requirements
|
|
Click |
Sequencing
|
| Bioinformatics Analysis We provide customized bioinformatics analysis including:
|
Analysis Pipeline
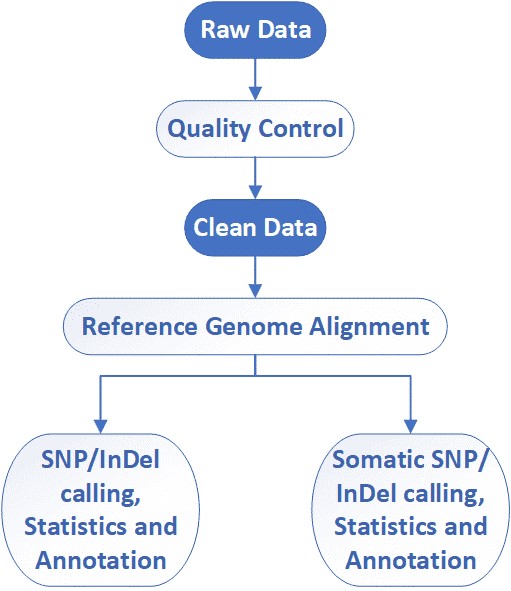
Deliverables
- The original sequencing data
- Experimental results
- Data analysis report
- Details in Whole Exome Sequencing for your writing (customization)
CD Genomics provides full whole exome sequencing service package including sample standardization, exome capture, library construction, deep sequencing, raw data quality control, and bioinformatics analysis. We can tailor this pipeline to your research interest. If you have additional requirements or questions, please feel free to contact us.
Reference:
- Warr A, Robert C, Hume D, et al. Exome sequencing: current and future perspectives. G3: Genes, Genomes, Genetics, 2015, 5(8): 1543-1550.
1. What are the applications of whole exome sequencing?
Human genome contains approximately 180,000 coding regions (exome), constituting about 1.7% of a human genome. It is estimated that 85% of the disease-causing mutations occur in the exome. Therefore, whole exome sequencing is a potential contributor to the understanding of human diseases. Whole exome sequencing is a cost-effective and powerful tool, especially suitable for bigger sample size and high coverage. Whole exome sequencing is mainly used to investigate the genetic cause of both Mendelian and common diseases such as cancer and diabetes.
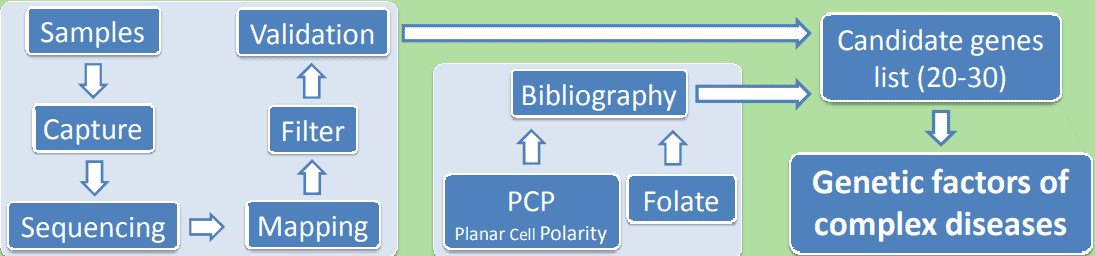 Figure 1. Application of whole exome sequencing in a complex disease.
Figure 1. Application of whole exome sequencing in a complex disease.
2. What variations can whole exome sequencing detect?
Whole exome sequencing can detect SNPs, InDels, and maybe copy number variations (CNVs).
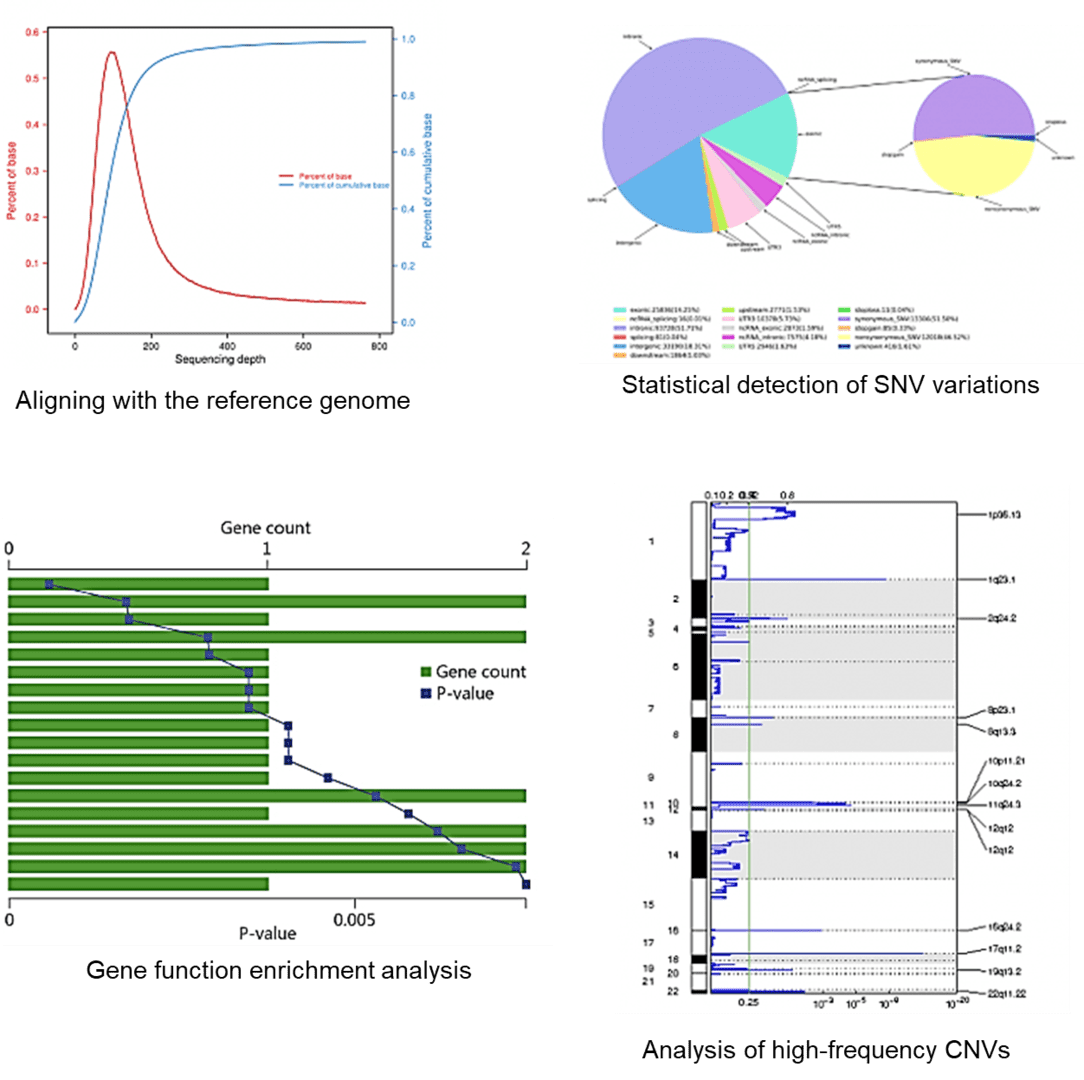
3. How do I determine the sequencing depth?
Sequencing depth is an important factor for high-throughput sequencing. One paper published in the journal Genomics & Informatics revealed that the sequencing depth of whole exome sequencing can affect the discovery rates of variations. To summarize, the number of deleterious SNPs and InDels detected in the coding regions was only weakly increased a depths more than 120×. In other words, a sequencing depth of 120× can be considered reasonable when using the exome capture sequencing technique to identify significant variations in diagnostic studies.
4. What are the disadvantages of whole exome sequencing?
Whole exome sequencing is characterized by lower cost, increased sequence coverage, as well as sensitive and specific identification. Nevertheless, Whole exome sequencing cannot detect structural variants, and has a limited view, i.e., only coding regions. Not all targets are captured (approximately 80%), and it is difficult to capture GC-rich regions.
Reference:
- Kyung Kim, et al. Effect of Next-Generation Exome Sequencing Depth for Discovery of Diagnostic Variants. Genomics & Informatics. 2015, Jun; 13(2): 31–39.
First missense mutation outside of SERAC1 lipase domain affecting intracellular cholesterol trafficking
Journal: Nuerogenetics
Impact factor: 3.269
Published online: 7 October 2015
Abstract
MEGDEL syndrome is a rare inborn error of metabolism. This syndrome has been associated to mutations in the serine active site containing 1 (SERAC1) gene. The authors reported a new homozygous mutation in the SERAC1 gene via whole exome sequencing at CD Genomics. This is the first missense mutation outside of serine-lipase domain of the protein that affects the intracellular cholesterol trafficking.
Results
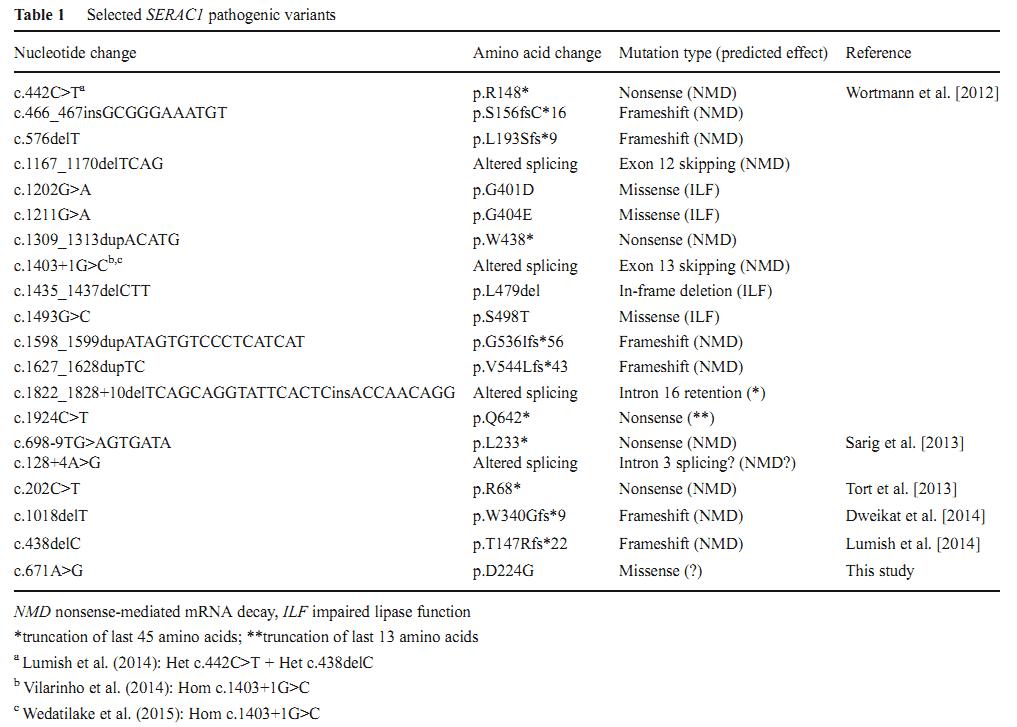
1. Mutations in the SERAC1 gene
To date, 19 mutations in the SERAC1 gene have been identified in patients with MEGDEL syndrome (Table 1). Only three are missense mutations which are localized within the lipase domain. The p.D224G is the first missense mutation outside of lipase domain.
2. Missense mutation (p. D224G)
Using whole exome sequencing, the authors identified a novel pathogenic homozygous mutation in the SERAC1 gene. This missense mutation changed a aspartic acid to glycine (Figure 1d). The pathogenic role of p.D224G is supported by in silico analysis, the conservation of the mutant amino acid residue (Figure 1d), and the accumulation of cholesterol (Figure 1e).
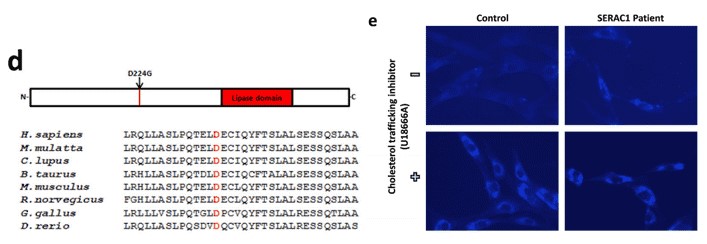 Figure 1. The position of D224 mutation in various species (d). Intracellular cholesterol trafficking in fibroblasts derived from healthy individual and the SERAC1 patients. U1866A is an inhibitor of cholesterol trafficking.
Figure 1. The position of D224 mutation in various species (d). Intracellular cholesterol trafficking in fibroblasts derived from healthy individual and the SERAC1 patients. U1866A is an inhibitor of cholesterol trafficking.
Reference:
- Rodríguez-García M E, et al. First missense mutation outside of SERAC1 lipase domain affecting intracellular cholesterol trafficking. Neurogenetics, 2016, 17(1): 51-56.
Here are some publications that have been successfully published using our services or other related services:
Optical Genome and Epigenome Mapping of Clear Cell Renal Cell Carcinoma
Journal: bioRxiv
Year: 2022
An independent origin of an annual life cycle in a North American killifish species
Journal: Biological Journal of the Linnean Society
Year: 2024
Combinations of Bacteriophage Are Efficacious against Multidrug-Resistant Pseudomonas aeruginosa and Enhance Sensitivity to Carbapenem Antibiotics
Journal: Viruses
Year: 2024
Genome sequence, antibiotic resistance genes, and plasmids in a monophasic variant of Salmonella typhimurium isolated from retail pork
Journal: Microbiology Resource Announcements
Year: 2024
High-Density Mapping and Candidate Gene Analysis of Pl18 and Pl20 in Sunflower by Whole-Genome Resequencing
Journal: International Journal of Molecular Sciences
Year: 2020
Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI
Journal: New phytologist
Year: 2017
See more articles published by our clients.


 Sample Submission Guidelines
Sample Submission Guidelines
