We use cookies to understand how you use our site and to improve the overall user experience. This includes personalizing content and advertising. Read our Privacy Policy

We are dedicated to providing outstanding customer service and being reachable at all times.
Overview of Mitochondrial DNA
At a glance:
- 1. Introduction
- 2. Mitochondrial DNA Mutations and Disease
- 3. Methods for Mitochondrial DNA Research
- 4. Applications of mtDNA Research
- 5. Future Prospects in mtDNA Research
1. Introduction
Mitochondrial DNA (mtDNA) is the genome found within mitochondria, the cell organelles, and is independently regulated to encode specific proteins. Unlike nuclear DNA, mtDNA has unique structural, genetic, and mutation characteristics.This article will explore the fundamental characteristics of mtDNA, its mutations and associated diseases, research methods, and its applications in biology and medicine, followed by a discussion on future research trends and directions.
1.1 Definition of Mitochondrial DNA
mtDNA is the second set of genetic material in eukaryotic organisms, distinct from the nuclear genome, and exhibits semi-autonomy, maternal inheritance, and low recombination rates. The origin of mitochondrial DNA traces back to the endosymbiotic theory, which proposes that mitochondria evolved by engulfing an ancient aerobic bacterium. Through this symbiotic relationship, the mitochondrial genome fused with the host's genome, ultimately forming a cellular structure with dual genomes.
1.2 Structure of Mitochondrial DNA
Mitochondrial genomic material in most animal species exists as a circular, duplex nucleic acid configuration. Specifically, the genetic blueprint within these cellular organelles encodes critical proteins and ribonucleic acid sequences. Genomic dimensions typically span approximately 14-20 kilobase segments, with the human mitochondrial chromosome measuring precisely 16,569 base pairs. The genetic composition encompasses a diverse array of molecular components: thirteen protein-encoding sequences, two ribosomal RNA genes, twenty-two transfer RNA elements, and a regulatory non-coding segment referred to as the displacement loop. Remarkably, these genetic instructions demonstrate unique characteristics—all genes lack interspersed non-coding regions and display overlapping genetic arrangements. The encoded genetic information primarily supports fundamental energetic processes, particularly oxidative phosphorylation mechanisms. In contrast to nuclear chromosomal structures, mitochondrial genetic material remains unassociated with histone proteins, resulting in a comparatively streamlined molecular organization.
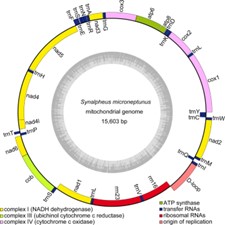 Figure1. Animal mitochondrial genome circle. (Chak,et.al ,2020)
Figure1. Animal mitochondrial genome circle. (Chak,et.al ,2020)
In plants, mtDNA is more complex, with mitochondrial genomes having single or multiple ring structures, linear branches, or even topological configurations that are difficult to analyze. Genome sizes vary significantly, ranging from 100 kb to 10 Mb, and most of the genome consists of non-coding sequences. A large proportion of the genome is made up of homologous sequences (2%-60%), with large intergenic regions that complicate genome assembly and annotation. Plant mtDNA not only contains genes for energy synthesis but also numerous genes related to unique plant physiological processes. These genes often exhibit a high mutation rate and are closely linked to metabolic regulation.
 Figure2. Plant mitochondrial genome circle. (Park,et.al ,2014)
Figure2. Plant mitochondrial genome circle. (Park,et.al ,2014)
1.3 Characteristics of Mitochondrial DNA
- Unique Genetic Features: The most notable feature of mtDNA is its maternal inheritance. In most species, mtDNA is passed down from the mother, though in rare cases, paternal mtDNA can be transmitted to offspring. This makes mtDNA an ideal tool for tracing maternal lineages.
- Higher Replication and Mutation Rates: mtDNA has a mutation rate significantly higher than that of nuclear DNA. The mitochondria's low DNA repair mechanisms make it more susceptible to environmental factors. These mutations are often the cause of various genetic disorders.
- Polymorphism in Plant mtDNA: Higher plant mitochondrial genomes contain numerous repetitive sequences, which account for 6.84%-58.34% of the entire mitochondrial genome. These sequences include both long and short repeats, with long repeats further categorized into direct and inverted repeats, with direct repeats being more common. Genes located within these long repeats become multi-copy genes.
1.4 Human mtDNA vs. Nuclear DNA
he following table provides a more detailed comparison between human mtDNA and nuclear DNA.
| Feature | Mitochondrial DNA (mtDNA) | Nuclear DNA (nDNA) |
|---|---|---|
| Shape | Circular DNA | Linear DNA |
| Size | Small (~16,500 base pairs) | Large (~3,200,000,000 base pairs) |
| Gene Structure | No introns; continuous genes | Genes contain introns; fragmented genes |
| Gene Coding | Both strands can encode genes | Only one strand encodes genes |
| Gene Overlap | Contains overlapping genes | No gene overlap |
| Intergenic Region | Short (1-30 base pairs) | Long intergenic regions, often much larger than the gene itself |
| Mutation Rate | Higher (10 times higher than nDNA) | Lower mutation rate |
| DNA Replication | Single-origin, unidirectional replication, continuous replication | Multiple origins, bidirectional replication |
| Replication Synchronization | Replicates independently from nuclear DNA, may replicate multiple times in a cycle | Replicates once per cycle, synchronized within the S phase |
| Transcription | Multisubunit promoters, often bidirectional; polycistronic mRNA | Unidirectional promoters; monocistronic mRNA |
| Codon Usage | UGA codes for tryptophan; four stop codons (UAA, UAG, UGA, AGA) | UGA, UAA, UAG are stop codons; AUG codes for methionine |
| Start Codon | AUG, AUA, AUU, AUC (methionine) or GUG (special case) | AUG (methionine) |
| tRNA Variety | Limited to 22 tRNA types | Extensive tRNA repertoire |
2. Mitochondrial DNA Mutations and Disease
2.1 Common Mitochondrial Diseases
Mitochondrial DNA mutations are closely associated with various genetic disorders. Below are some of the most common mitochondrial diseases:
Mitochondrial Myopathy
Mitochondrial myopathy represents a cluster of pathological conditions characterized by compromised muscular performance. Patients typically experience profound muscular debilitation, characterized by progressive weakness, cellular muscle deterioration, and persistent energy depletion. These neurological and muscular disorders stem from fundamental disruptions in mitochondrial energetic processes, resulting in inadequate adenosine triphosphate generation for muscular cellular requirements. Genetic alterations frequently manifest in specific genomic regions, notably the MT-TL1 and MT-ATP6 genetic loci.
Leber's Hereditary Optic Neuropathy (LHON)
Characterized as a distinctive retinal degenerative condition, LHON emerges through mitochondrial genetic modifications. The disorder predominantly impacts young male populations, precipitating abrupt visual function deterioration. Specific mitochondrial genetic variations within ND1, ND4, and ND6 sequences significantly disrupt cellular electron transportation mechanisms, compromising oxidative phosphorylation and subsequently undermining optical nerve energetic infrastructure.
Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS)
MELAS represents a comprehensive mitochondrial dysfunction syndrome predominantly affecting neurological and muscular systems. Clinically, patients demonstrate complex symptomatology including metabolic acidification and neurological events resembling cerebrovascular incidents. Genetic modifications within the MT-TL1 gene fundamentally compromise mitochondrial energetic production, precipitating progressive neurodegenerative transformations and metabolic lactate accumulation.
Kearns-Sayre Syndrome (KSS)
Kearns-Sayre Syndrome emerges as an exceedingly rare mitochondrial genomic disorder. Clinical manifestations encompass external ocular muscle paralysis, cardiac electrical conduction irregularities, and multisystemic physiological complications. The syndrome predominantly originates from extensive mitochondrial genome deletions, systematically disrupting critical genetic regulatory mechanisms.
Chronic Progressive External Ophthalmoplegia (CPEO)
CPEO manifests as a persistent, incrementally deteriorating ocular muscular paralysis syndrome. The condition predominantly impacts extraocular muscular functionality, presenting with progressive muscular weakness and compromised visual motor control. Genetic variations within mitochondrial DNA, including extensive rearrangements and localized deletions, serve as primary etiological mechanisms underlying this complex neuromuscular disorder.
2.2 How mtDNA Mutations Lead to Disease
mtDNA mutations often result in electron transport chain (ETC) dysfunction, impairing ATP synthesis. Recent studies indicate that some mtDNA mutations may disrupt enzyme functions in oxidative phosphorylation, leading to inadequate cellular energy production and triggering a series of metabolic and tissue damages. Additionally, mtDNA mutations can cause mitochondrial dysfunction, contributing to neurodegenerative diseases such as Alzheimer's disease.
3. Methods for Mitochondrial DNA Research
3.1 Mitochondrial DNA Sequencing Technologies
Several major strategies for mitochondrial DNA sequencing can be summarized as follows:
1. PCR-based Sequencing Strategy
This method first uses universal primers to amplify conserved short fragments in the mitochondrial genome. Specific primers are then designed based on these fragments, and long-PCR techniques are used to divide mtDNA into overlapping long fragments. These PCR products are subsequently trimmed using physical methods to obtain appropriately sized fragments for high-throughput sequencing. This strategy simplifies the assembly process and yields high accuracy in sequencing. However, due to primer limitations, obtaining long PCR products can be challenging, potentially affecting the coverage of certain regions.
2. Target Capture Sequencing Strategy
Target capture is a recently developed method for high-throughput sequencing, commonly using a microarray-based mtDNA hybridization enrichment method. Large numbers of oligonucleotide probes are synthesized, which can complementarily bind with the mitochondrial genome to enrich mtDNA fragments. After enrichment, these mtDNA fragments undergo high-throughput sequencing, providing detailed information about the entire mitochondrial genome. This strategy reduces PCR steps and minimizes the chances of introducing errors, making it particularly suitable for complex mitochondrial genome variant analysis.
3. mtDNA Extraction, Purification, and Sequencing Strategy
Methods for extracting mtDNA include cesium chloride ultracentrifugation, column chromatography, and alkaline denaturation methods. However, because mtDNA constitutes a small portion of total cellular DNA, and its extraction requires fresh tissues, organs, or individuals, the extraction process is time-consuming and yields relatively low amounts. To improve extraction efficiency, Quispe-Tintaya et al. designed a rapid method to isolate mtDNA from mammalian cells, achieving about 2000-fold enrichment. The enriched mtDNA is then sequenced using high-throughput sequencing (NGS), improving experimental efficiency and data quality. Recently, single-cell sequencing and long-read sequencing have provided new solutions for addressing the structural complexity of mtDNA. At present, human mitochondrial sequencing and animal and plant mitochondrial sequencing are the research hotspots.
3.2 Mitochondrial DNA Data Analysis
With continuous advancements in sequencing technologies, tools and software for analyzing mtDNA data have significantly improved. mtDNA sequencing data analysis involves genome assembly and annotation, genomic composition analysis, sequence submission, and visualization. Common tools such as Staden Package, MITOS, and CGview are used for accurate assembly and gene annotation, as well as analyzing gene content and nucleotide composition. Advanced analytical methods include phylogenetic tree construction, selective pressure analysis, genome polymorphism analysis, and Simple Sequence Repeat (SSR) analysis, which help explore evolutionary relationships and gene functions. RNA editing analysis and tRNA secondary structure analysis are also utilized to better understand mitochondrial gene expression and adaptation mechanisms, advancing species classification and evolutionary studies.
4. Applications of mtDNA Research
- Species Classification and Identification: Due to its higher mutation rate and maternal inheritance, mtDNA is widely used in species classification, identification, and evolutionary studies.
- Animal Population Genetics and Molecular Phylogenetics: mtDNA is frequently used as a genetic marker for studying species diversity, population structure, and evolutionary history.
- Human mtDNA Sequencing Applications: These include mitochondrial diseases, human identity testing, forensic science, and cancer research. mtDNA mutations are closely associated with several mitochondrial diseases, and mtDNA serves as an important biomarker in forensic and cancer research.
- Genetic Variation Analysis in Geographic Populations: By analyzing the mitochondrial genomes of different geographic populations, researchers can explore the relationship between geographic distribution and genetic variation.
5. Future Prospects in mtDNA Research
Contemporary mitochondrial genomic investigations are progressively converging across multiple scientific disciplines, propelled by transformative technological innovations. Emerging research trajectories encompass several critical domains:
Advanced Genomic Sequencing Methodologies: Cutting-edge sequencing approaches, including extended-read and tertiary-generation technologies, demonstrate promising potential for unraveling intricate mitochondrial genetic architectures. These sophisticated techniques will facilitate comprehensive mapping of complex genomic structures with unprecedented resolution.
Sophisticated Mutation Diagnostic Strategies: Integration of computational intelligence and advanced algorithmic learning paradigms is anticipated to revolutionize mtDNA mutation identification. These innovative analytical frameworks will significantly enhance diagnostic precision, enabling more nuanced characterization of genetic variations.
Comprehensive Mitochondrial Pathogenesis Investigations: Future scientific endeavors will systematically explore intricate relationships between mitochondrial genetic modifications and complex pathological mechanisms. Researchers aim to elucidate molecular pathways connecting genetic aberrations with multisystemic disease manifestations, potentially identifying novel therapeutic interventions.
As investigative methodologies continue to evolve, our comprehension of mitochondrial genetic complexity will exponentially expand. Anticipated breakthroughs will substantially illuminate fundamental genetic mechanisms, generating transformative insights across interdisciplinary domains including biomedical research, clinical diagnostics, molecular medicine, and forensic sciences.
References
- Quispe-Tintaya, et,al. (2013). Fast mitochondrial DNA isolation from mammalian cells for next-generation sequencing.BioTechniques, 55(3), 133–136. https://doi.org/10.2144/000114077
- Chak,et.al. (2020). The complete mitochondrial genome of the eusocial sponge-dwelling snapping shrimp Synalpheus microneptunus. Scientific reports, 10(1), 7744. https://doi.org/10.1038/s41598-020-64269-w
- Park,et.al. (2014). Complete sequences of organelle genomes from the medicinal plant Rhazya stricta (Apocynaceae) and contrasting patterns of mitochondrial genome evolution across asterids. BMC genomics, 15(1), 405. https://doi.org/10.1186/1471-2164-15-405
For research purposes only, not intended for personal diagnosis, clinical testing, or health assessment