We use cookies to understand how you use our site and to improve the overall user experience. This includes personalizing content and advertising. Read our Privacy Policy

We are dedicated to providing outstanding customer service and being reachable at all times.
Short Telomeres Syndrome: Symptoms, Causes, and Treatment Options
At a glance:
- Telomeres and Their Role in Health
- Mechanisms of telomere shortening
- Causes of Short Telomere Syndrome
- Symptoms and Clinical Manifestations
- Diagnosis and Telomere Length Testing
- Treatment and Management Options
- Conclusion
Telomere length deficiency disorders, collectively known as Short Telomere Syndrome (STS), encompass various hereditary conditions stemming from genetic alterations affecting telomere biology. This disorder manifests through accelerated reduction in telomere length, which results in early cellular senescence and impaired function. The impact of STS extends beyond developmental challenges, as it demonstrates significant associations with numerous age-related pathologies. The underlying mechanism of this inherited condition primarily involves defects in genes responsible for preserving telomeric regions. When these crucial genetic components undergo alterations, they trigger rapid telomere erosion and premature aging at the cellular level, manifesting in diverse clinical presentations. Advancing our understanding of STS pathophysiology and developing novel therapeutic approaches holds promise not only for enhancing outcomes in affected individuals but may also illuminate potential interventions for broader age-associated medical conditions.
Telomeres and Their Role in Health
Structure and function of telomeres
Telomeres are structures made up of repeated DNA sequences, such as TTAGGG, that shorten each time a cell divides. The main function of telomeres is to prevent fusion between chromosome ends, thereby protecting the integrity of the genome. When telomeres shorten to a certain extent, cells will enter replicative senescence, eventually leading to cell death.
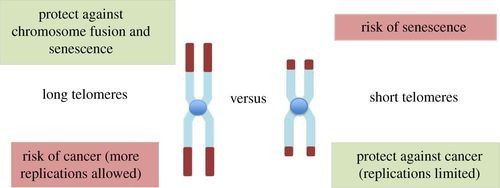 Figure 1. Opposite effects of long and short telomeres.(Risques, R. A., et.al,2018)
Figure 1. Opposite effects of long and short telomeres.(Risques, R. A., et.al,2018)
Mechanisms of telomere shortening
The main mechanisms of telomere shortening include: decreased telomerase activity, oxidative stress and genetic mutations. Telomerase is an enzyme that lengthens telomeres, but in most somatic cells, telomerase activity is low or completely absent. Therefore, as the number of cell divisions increases, telomeres gradually shorten. Oxidative stress can accelerate telomere shortening, especially in certain disease states such as chronic inflammation and tumors. Changes in gene expression in the telomere region may also affect telomere length.
Causes of Short Telomere Syndrome
Genetic mutations
The pathogenesis of short telomere syndrome is mainly related to mutations in genes that maintain telomere length. These genes include:
TERT: The reverse transcriptase subunit that encodes telomerase and is a key component of telomerase activity. Mutations in the TERT gene can lead to a significant decrease in telomerase activity, making cells unable to effectively maintain telomere length, thus causing clinical symptoms of STS.
TERC: RNA template that encodes telomerase and provides a template for telomerase to synthesize telomeric DNA. Mutations in the TERC gene cause a decrease in telomerase activity, which in turn accelerates telomere shortening.
DKC1: Encoding the dyskerin protein, involved in the assembly and stability of telomerase. Mutations in the DKC1 gene affect the assembly and function of telomerase, leading to shortening of telomeres.
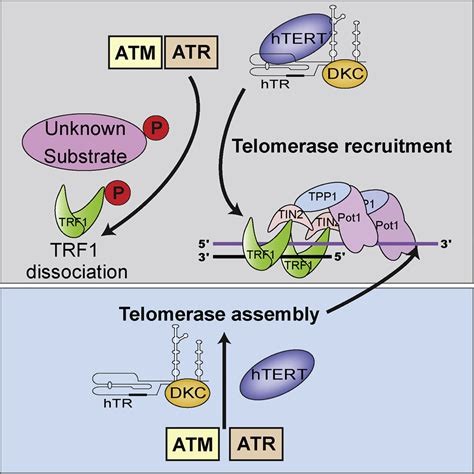 Figure 2. ATM and ATR Signaling.(Tong, A. S., et.al,2015)
Figure 2. ATM and ATR Signaling.(Tong, A. S., et.al,2015)
TINF2: Encoding the TRF1-binding protein that regulates telomere length and structure.
NHP2, NOP10: Involved in the assembly and function of telomerase complex.
Mutations in these genes can lead to decreased telomerase activity or abnormal telomere structure, which in turn accelerates telomere shortening. For example, mutations in the TERT gene can lead to a significant decrease in telomerase activity, making cells unable to effectively maintain telomere length, thereby triggering clinical symptoms of STS.
Environmental and lifestyle factors
In addition to genetic mutations, environmental and lifestyle factors can also accelerate telomere shortening. These factors include:
Oxidative stress: Long-term exposure to high oxidative stress environments, such as smoking, air pollution, chronic inflammation, etc., increases the risk of telomere damage.
Psychological stress: Long-term psychological stress can activate the stress response, leading to increased oxidative stress and accelerating telomere shortening.
Bad eating habits: High-sugar, high-fat diets and diets lacking antioxidants can increase oxidative stress and affect telomere length.
Lack of exercise: Regular physical exercise helps maintain telomere length, while lack of exercise accelerates telomere shortening.
Clonal hematopoiesis and its link to short telomeres
Clonal Hematopoiesis (CHIP) refers to the emergence of somatic mutations in hematopoietic stem cells, resulting in the proliferation of clonal cells. The incidence of CHIP in patients with short telomere syndrome is significantly higher than that in the general population. These mutations usually involve genes such as TET2 and TP53 and are closely related to the development of hematological malignancies. Studies have shown that the emergence of CHIP-related mutations in patients with short telomeres may be related to cellular aging and DNA damage repair defects caused by telomere shortening (Yamaguchi, H., et.al,2005). Telomere shortening activates the DNA damage response, inducing cell cycle arrest and apoptosis, and certain somatic mutations can escape these cell fate determining mechanisms, thereby promoting clonal cell proliferation. Therefore, CHIP is not only a prelude to hematological malignancies in short telomere syndrome, but may also participate in pathological processes in other tissues.
Symptoms and Clinical Manifestations
Hematological disorders
Patients with short telomere syndrome often present with hematological diseases, mainly including:
Bone marrow failure: Due to shortening of telomeres of hematopoietic stem cells, the cells age prematurely, resulting in a decline in bone marrow hematopoietic function. Patients often present with anemia, a tendency to bleed, and an increased risk of infection.
Myelodysplastic syndrome (MDS): DNA damage and cell cycle arrest caused by telomere shortening can trigger abnormal clonal proliferation of bone marrow cells. Patients with MDS often present with peripheral blood cytopenia, abnormal bone marrow cell morphology, and chromosomal abnormalities (such as monomer 7).
Acute myeloid leukemia (AML): Some patients with MDS will progress to AML, and telomere shortening accelerates the clonal proliferation of leukemia cells and the accumulation of genetic mutations. Patients with AML often present with high white blood cell counts, anemia, bleeding, and infection.
Pulmonary conditions
Among patients with short telomere syndrome, lung disease is one of the common clinical manifestations, mainly including idiopathic pulmonary fibrosis (IPF). Telomere shortening can lead to premature aging and apoptosis of alveolar cells, which in turn leads to abnormal repair and fibrosis of lung tissue. Patients with IPF often present with progressive dyspnea, dry cough, and reduced lung function. Pathological examination showed destruction of the alveolar structure, thickening of the alveolar septum, and increased collagen deposition. Studies have shown that telomere shortening not only affects alveolar epithelial cells, but also affects the function of alveolar macrophages and fibroblasts, and together promotes the occurrence and development of pulmonary fibrosis.
Liver diseases
Patients with short telomere syndrome also often present with liver diseases, mainly including liver fibrosis and liver cirrhosis. Telomere shortening can lead to premature aging and apoptosis of liver cells, which in turn triggers liver inflammation and fibrosis. Patients with liver fibrosis often present with abnormal liver function, abdominal distension and fatigue. As the disease progresses, some patients will develop liver cirrhosis and develop complications such as ascites, jaundice and hepatic encephalopathy. Pathological examination showed liver cell degeneration and necrosis, liver fibrous tissue proliferation, and liver lobular structure disorder. Studies have shown that telomere shortening activates hepatic stellate cells, promotes collagen synthesis and deposition, thereby accelerating the process of liver fibrosis.
Cancer risks
Patients with short telomere syndrome have a significantly increased risk of cancer, mainly including myeloid malignancies and squamous cell carcinomas. Telomere shortening increases the probability of chromosome ends fusing, leading to chromosome aneuploidy and translocations, thereby promoting tumor development. The pathogenesis of myeloid malignancies is related to telomere shortening and DNA damage repair defects in bone marrow cells. Patients often present with abnormal clonal proliferation and accumulation of gene mutations in bone marrow cells. Squamous cell carcinoma mostly occurs in the skin and mucosa. Telomere shortening can lead to premature aging and apoptosis of skin and mucosa epithelial cells, which in turn leads to abnormal cell proliferation and cancer. Studies have shown that patients with squamous cell carcinoma often carry mutations in TP53 and TERT promoter region, which help maintain telomere length and promote the clonal proliferation of tumor cells.
Immunodeficiency
Patients with short telomere syndrome often present with immunodeficiency, mainly including T-cell immunodeficiency. Telomere shortening can lead to premature aging and apoptosis of T cells, which in turn affects T cell proliferation and function. Patients often present with repeated infections, autoimmune diseases, and increased susceptibility to tumors. Studies have shown that T cell immune deficiency is closely related to the risk of solid tumors in patients with short telomere syndrome. T cell dysfunction can weaken the body's ability to immune monitor tumor cells, thereby promoting the occurrence and development of tumors. In addition, immune deficiencies can lead to reduced resistance to infection and increase the risk of infection-related complications.
Diagnosis and Telomere Length Testing
Methods of measuring telomere length
Telomere length measurement is an important method for diagnosing short telomere syndrome. Common measurement methods include:
Flow cytometry combined with fluorescence in situ hybridization (flowFISH): Using fluorescently labeled telomere probes, combined with flow cytometry, the telomere length of peripheral blood cells is quantitatively measured. This method has the characteristics of high throughput and high sensitivity and is suitable for large-scale screening.
Quantitative polymerase chain reaction (qPCR): Telomere length is calculated by comparing the relative content of telomere DNA to a single copy of the gene. This method is simple and low-cost, but requires standardized reference genes.
Southern blot: Telomere DNA fragments are separated by restriction enzyme digestion and gel electrophoresis, combined with radioactive or fluorescently labeled telomere probes for hybridization, and telomere length is measured. This method has high resolution, but the operation is complex and the cost is high.
Genetic testing for telomere biology disorders
Genetic testing is a key step in the diagnosis of short telomere syndrome. By detecting genetic mutations related to telomere maintenance, the cause of disease can be clarified. Common genes tested include TERT, TERC, DKC1, TINF2, etc. Genetic testing methods include:
Whole exon sequencing (WES): detects gene mutations in the entire exon region and is suitable for large-scale genetic screening.
Targeted gene sequencing: Deep sequencing of specific genes to detect known pathogenic mutations has high detection efficiency.
Multiple ligation probe amplification (MLPA): detects gene deletions or duplications and is suitable for detecting gene structural variations.
Treatment and Management Options
Hematological Interventions
Bone marrow and stem cell transplantation: This is currently one of the main methods for treating STS. By transplanting healthy hematopoietic stem cells, patients 'hematopoietic function can be restored. However, this approach carries certain risks, such as graft-versus-host disease (GVHD) and the risk of infection.
Pulmonary and Liver Disease Management
For patients with pulmonary fibrosis, anti-fibrotic drugs may help slow the progression of the disease. However, there is currently no specific treatment for pulmonary fibrosis. Liver transplants may be necessary for patients with severely impaired liver function. Studies have shown that liver transplantation can improve survival in STS patients, but it is important to note that the use of immunosuppressants may accelerate telomere shortening.
Emerging Therapeutic Approaches
Activation of telomerase (such as hTERT) through gene therapy can lengthen telomeres, thereby delaying cell aging and disease progression. Research has shown that mesenchymal stem cell (MSC) transplants may help repair damaged tissues and organs. Adjust the immunosuppression regimen and avoid the use of T cell depleting agents to reduce immune-related complications.
Conclusion
The clinical manifestations of STS are diverse and involve multiple systems, including hematological diseases, lung diseases, liver diseases, increased risk of cancer, and immune deficiencies. Diagnosis of STS requires a combination of telomere length measurement and genetic testing to clarify the cause. Treating STS requires comprehensive management, including blood system intervention, lung and liver disease management, cancer surveillance and prevention, potential applications of immunotherapy, and emerging treatment strategies.
With the deepening of research on telomere biology, treatment and prevention strategies for short telomere syndrome have been continuously optimized. Emerging treatment strategies such as gene editing technology, telomerase activation strategies, and research drugs targeting the telomere maintenance pathway provide new hope for the treatment of STS. At the same time, telomere supplements and lifestyle adjustments also provide new ways to delay telomere shortening. Future research will further deepen the understanding of telomere biology, develop more effective treatment and prevention strategies, provide better outcomes for STS patients and improve their quality of life.
References
- Risques, R. A., & Promislow, D. E. L. (2018). All's well that ends well: why large species have short telomeres. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1741). https://doi.org/10.1098/rstb.2016.0448
- Yamaguchi, H., Calado, R. T., Ly, H., Kajigaya, S., Baerlocher, G. M., Chanock, S. J., Lansdorp, P. M., & Young, N. S. (2005). Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. The New England journal of medicine, 352(14), 1413–1424. https://doi.org/10.1056/NEJMoa042980
- Tong, A. S., Stern, J. L., Sfeir, A., Kartawinata, M., de Lange, T., Zhu, X. D., & Bryan, T. M. (2015). ATM and ATR Signaling Regulate the Recruitment of Human Telomerase to Telomeres. Cell reports, 13(8), 1633–1646. https://doi.org/10.1016/j.celrep.2015.10.041
For research purposes only, not intended for personal diagnosis, clinical testing, or health assessment